The following data were obtained by gas-liquid chromatography on a 40-cm packed column: Calculate (a) An average
Question:
The following data were obtained by gas-liquid chromatography on a 40-cm packed column:
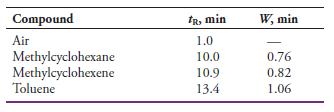
Calculate
(a) An average number of plates from the data.
(b) The standard deviation for the average in (a).
(c) An average plate height for the column.
Transcribed Image Text:
Compound IR, min W, min Air 1.0 Methylcyclohexane Methylcyclohexene Toluene 10.0 0.76 10.9 0.82 13.4 1.06
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
The number of plates N for a chromatograph is calculated as N 554 x t ...View the full answer

Answered By

Manmohan V
First of all, I have devoted five years to gain the knowledge of chemistry bottom-up at my University. After my undergraduate, I started teaching and tutoring as well on some online platforms. I have worked as a Subject Metter Expert with three companies and gained perfection in Microsoft Office, Latex, ChemDraw and many online tools to deliver fast and accurate solutions.
As a freelancer, I am working on two online portals, where I have solved over 4000 queries in the past two years with a perfect quality score.
With my Creative skills of explanation, I guarantee that you will not face any problems with the solutions provided by me.
Thanks.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:
Students also viewed these Sciences questions
-
In a recent survey, the following data were obtained in response to the question, If the number of summer classes were increased, would you be more likely to enroll in one or more of them? If a...
-
The following data were obtained when a cold-worked metal was annealed. (a) Estimate the recovery, recrystallization, and grain growth temperatures; (b) Recommend a suitable temperature for a...
-
Suppose that the following data were obtained by an investigator studying the influence of estrogen injections on change in the pulse rate of adolescent chimpanzees: a. What are the factors in this...
-
Which type of Organizational Model put the MOST emphasis on establishing MORALE and AUTONOMY O a. Hierarchical style Model b. O c. O d. Flat Management (Holocracy) Model Multi-divisional style Model...
-
A mortgage broker offers to sell you a mortgage loan contract that will pay $800 at the end of each month for the next 3 1/2 years, at which time the principal balance of $45,572 is due and...
-
Find the 90% confidence interval for the variance and standard deviation for the lifetimes of inexpensive wristwatches if a sample of 24 watches has a standard deviation of 4.8 months. Assume the...
-
[Appendix] Prepare balance sheets of pooled companies Pop Corporation issued its own common stock for all the outstanding shares of Son Corporation in a pooling of interests business combination on...
-
In the current year, Madison Corporation had $50,000 of taxable income at a tax rate of 25%. During the year, Madison began offering warranties on its products and has a warranty liability for...
-
A company's inventory records report the following in November of the current year: Units Sold at Retail Date November 1 November 2 November 8 November 12 Activities Beginning inventory Purchase...
-
Distinguish between taxes that are proportional and those that are progressive.
-
An open tubular column used for gas chromatography had an inside diameter of 0.15 mm. A volumetric flow rate of 0.85 mL/min was used. Find the linear flow velocity in cm/s at the column outlet.
-
Describe the preparation of exactly 1.00 L of 0.1000 M HCl from primary-standard-grade NaCl using a cation-exchange resin.
-
What are the implications of the issues raised so far in this chapter for your own learning and development needs? How would you attempt to meet these needs? In the light of this, what would you seek...
-
In your opinion, do you feel the Six Pillars apply primarily to business-to-consumer marketing, business-to-business marketing, or both?
-
Place yourself in context; You're working, trying to do well, running into all sorts of organizational constraints. First, describe a scenario to illustrate one of the eight organizational...
-
what ways do mindfulness-based stress reduction (MBSR) techniques contribute to reducing stress, and what are their limitations ?
-
How do search engines understand "user experience" to evaluate organic rankings?
-
How do you think a risk manager might work through a difference of opinion with a unit manager? Explain in details.
-
Assume Cramer uses the direct method to prepare the statement of cash flows. Credit sales totaled $750,000, accounts receivable increased by $40,000, and accounts payable decreased by $25,000. How...
-
A container holds 2.0 mol of gas. The total average kinetic energy of the gas molecules in the container is equal to the kinetic energy of an 8.0 10-3-kg bullet with a speed of 770 m/s. What is the...
-
Neglecting any effects caused by volume changes, would you expect the ionic strength to (1) increase, (2) decrease, or (3) remain essentially unchanged when NaOH is added to a dilute solution of (a)...
-
Explain why the activity coefficient for dissolved ions in water is usually less than that for water itself.
-
Explain why the initial slope for Ca2+ in Figure 10-3 is steeper than that for K+? 0.8 0.6 0.4 0.2 Fe(CN)64- 0 0 0.1 0.2 0.3 0.4
-
Discuss why it is important for company managers to understand and use social capital knowledge to help build social ties among their skilled knowledge workers so they can build employee loyalty...
-
Kate lives in a house close to a local university, and she traditionally has rented a garage apartment in the back of her property to students for $750 per month. Kate wants to transfer the title to...
-
Pottery Ranch Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production at...

Study smarter with the SolutionInn App


