Titration of 50.00 mL of 0.04715 M Na 2 C 2 O 4 required 39.25 mL of
Question:
Titration of 50.00 mL of 0.04715 M Na2C2O4 required 39.25 mL of a potassium permanganate solution.
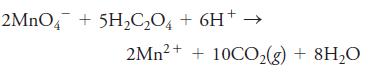
Calculate the molar concentration of the KMnO4 solution.
Transcribed Image Text:
2MNO4 + 5H2C,O, + 6H+ 2Mn?+ + 10CO,(g) + 8H20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 22% (9 reviews)
For this redox titration we use the formu...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:
Students also viewed these Sciences questions
-
Calculate the molar concentration of a solution that is 50.0% NaOH (w/w) and has a specific gravity of 1.52.
-
Calculate the molar concentration of a 20.0% solution (w/w) of KCl that has a specific gravity of 1.13
-
Calculate the molar concentration of a dilute Ba(OH)2 solution if (a) 50.00 mL yielded 0.1791 g of BaSO4. (b) Titration of 0.4512 g of primary standard potassium hydrogen phthalate (KHP) required...
-
The pilot of a small boat charts a course such that the boat will always be equidistant from an upcoming rock and the shoreline. Describe the path of the boat. If the rock is 2 miles offshore, write...
-
Using the bond yield given in the final column of Table 15.2, verify the September 22, 2009, quoted price for the Province of New Brunswick 4.4% coupon bond, maturing June 3, 2019.
-
Jason attends his high school reunion. Of the attendees, 50% are female. Common knowledge has it that 88% of people are right-handed. Being a left handed male, Jason knows that of a given crowd, only...
-
Analysis of Variance for Response Source DF SS MS F P Regression 7 105.4375 15.0625 4.7255 0.0222 A 1 27.5625 27.5625 8.6471 0.0187 B 3 8.1875 2.7292 0.8562 0.5017 A*B 3 69.6875 23.2292 7.2876 0.0112...
-
You are normally an easygoing manager who gives your employees a lot of leeway in using their own personal communication styles. However, the weekly staff meeting this morning pushed you over the...
-
Current Attempt in Progress Bridgeport Corporation produces area rugs. The following unit cost information is available: direct materials $ 18, direct labor $ 10, variable manufacturing overhead $ 8,...
-
Franklin, Inc., accumulates large amounts of excess cash throughout the year. It typically invests these funds in marketable securities, both short term and long term. The companys most recent...
-
The phosphorus in a 0.3019-g sample was precipitated as the slightly soluble (NH 4 ) 3 PO 4 . 12MoO 3 . This precipitate was filtered, washed, and then redissolved in acid. Treatment of the resulting...
-
A 0.7891-g sample of a mixture consisting solely of sodium bromide and potassium bromide yields 1.2895 g of silver bromide. What are the percentages of the two salts in the sample?
-
Did Soldau create a contract by mailing the release? Organon fired John Soldau. Then the company sent to him a letter offering to pay him double the normal severance pay, provided Soldau would sign a...
-
The following information summarizes the activities in the Mixing Department for the month of March. Beginning inventory 1 , 0 0 0 units, 8 0 % complete Started and completed 2 4 , 5 0 0 units Ending...
-
What is your recommendation for the maximum size of coarse aggregate for the following situation? A continuously reinforced concrete pavement cross section contains a layer of No. 6 reinforced bars...
-
On January 1, 2024, Winn Heat Transfer leased office space under a three-year operating lease agreement. The arrangement specified three annual lease payments of $72,000 each, beginning December 31,...
-
A closed square pyramid tank (base width: 6.0 m; height 3.0 m), sitting on its square base, has a 1.0 m depth of water. Suppose this tank is inverted (turned upside down) and is made to stand on its...
-
P.4.3 Apply a Taylor series expansion to a mixed backward formula for the first derivative: (Ux)i = 1 Ax (aui-2+ bui-1 + cu + dui+1) Derive the family of second order accurate formulas and the...
-
How does the identification of key audit areas relate to the effectiveness and efficiency of an audit?
-
A handrail, which weighs 120 N and is 1.8 m long. was mounted to a wall adjacent to a small set of steps (Figure P4.26). The support at A has broken, and the rail has fallen about the loose bolt at 8...
-
Indicate whether an aqueous solution of the following compounds is acidic, neutral, or basic. Explain your answer. *(a) NH4OAc (b) NaNO2 *(c) NaNO3 (d) NaHC2O4 *(e) Na2C2O4 (f) Na2HPO4 *(g) NaH2PO4...
-
Suggest an indicator that could be used to provide an end point for the titration of the first proton in H3AsO4.
-
Suggest a method for determining the amounts of H3PO4 and NaH2PO4 in an aqueous solution.
-
Questien It Calraluta bae neark yoe cen atforal to berren
-
In calculating the net present value of a proposed project, the cash flows of the project should include a.) amortization of goodwill b.) interest expenses paid to bondholders c.) extra working...
-
If Yolanda's insurance company cancels her fire insurance policy after 204 days, how much of the $682.00 annual premium will she receive as a refund (in $)? (Round you answer to the nearest cent.) $

Study smarter with the SolutionInn App


