The reversible reaction shown here is part of the Calvin cycle, a pathway in photosynthetic organisms. Which
Question:
The reversible reaction shown here is part of the Calvin cycle, a pathway in photosynthetic organisms. Which glycolytic reaction does this reaction resemble and what type of enzyme catalyzes it?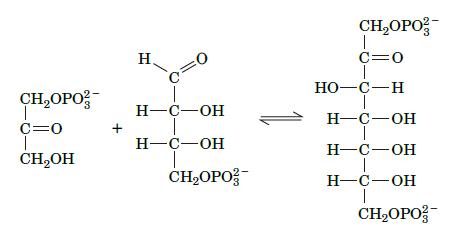
Transcribed Image Text:
CHOPO 1 C=0 CHOH + H =0 H-C-OH H-C-OH CHOPO CHOPO T C=0 HO-C-H H-C-OH H-C-OH H-C-OH T CHOPO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The reversible reaction shown in the Calvin cycle that resembles a glycolytic reaction is t...View the full answer

Answered By

BRIAN MUSINGA
I possess a Bachelors of Commerce degree(Marketing option) and am currently undertaking an MBA in marketing. I believe that I possess the required knowledge and skills to tutor in the subject named. I have also written numerous research academic papers much to the satisfaction of clients and my professors.
5.00+
2+ Reviews
17+ Question Solved
Related Book For 

Fundamentals Of Biochemistry Life At The Molecular Level
ISBN: 9781118918401
5th Edition
Authors: Donald Voet, Judith G Voet, Charlotte W Pratt
Question Posted:
Students also viewed these Sciences questions
-
Step 4 of the pentose phosphate pathway converts ribulose-5- hosphate to ribose-5-phosphate. Which glycolytic reaction does this reaction resemble and what type of enzyme catalyzes it?
-
The bacterial enzyme polynucleotide phosphorylase (PNPase) is a 3 S exoribonuclease that degrades mRNA. (a) The enzyme catalyzes a phosphorolysis reaction, as does glycogen phosphorylase (Section...
-
The Earth has been getting warmer. Most climate scientists agree that one important cause of the warming is the increase in atmospheric levels of carbon dioxide (CO2), a greenhouse gas. Here is part...
-
Identify possible opportunities for denormalizing these relations as part of the physical design of the database. Which ones would you be most likely to implement
-
The capital investment committee of Eastern Trucking Inc. is considering two investment projects. The estimated income from operations and net cash flows from each investment are as follows: Each...
-
What is credit quality, and how is it assessed? AppendixL01
-
What is the conceptual relationship between the discount rates at the marketable minority (Rmm) and nonmarketable minority (Rhp) levels of value?
-
Given the following business scenario, create a Crows Foot ERD using a specialization hierarchy if appropriate. Granite Sales Company keeps information on employees and the departments that they work...
-
Omega Tire Cos perpetual inventory rekords indicate that 53 145,000 of merchandise should be on hand on August 31, 2016. The chysical entory indicates that 31, 13.500 of merchandises actually on hand...
-
In a mixture of NAD+, NADH, ubiquinone, and ubiquinol, which compound will be oxidized? Which will be reduced?
-
The thioester described in Problem 29 reacts readily with compounds with the formula ROH or RNH2. Draw the resulting ester and amide reaction products. Problem 29 Some proteins contain internal...
-
In Problems 1928, analyze each equation. That is, find the center, vertices, and foci of each ellipse and graph it. X 9 4 = 1
-
Avery, an unmarried taxpayer, had the following income items: Salary Net income from a rental house 3 7 , 0 5 0 4 , 9 0 0 Avery has a 4 - year - old child who attends a child care center. Assume the...
-
California Lottery Let A denote the event of placing a $1 straight bet on the California Daily 4 lottery and winning. There are 10,000 different ways that you can select the four digits (with...
-
"Tamara Wiley glanced in the mirror before leaving her apartment and heading to her 8 a.m. class. She was having a bad hair day, so she had thrown on a scarf. Her quick check in the mirror told her...
-
Online Friends In a Pew Research Center survey of 1060 teens aged 13 to 17, it was found that 604 (or 57.0%) of those respondents have made new friends online. If the true rate is 50%, there is a...
-
Dr. Yong has requested that Senture Houston, an office manager at Pain Free Dental Associates, prepare a single journal entry for December 31, 2022. The bank statement for that day shows $9,500....
-
Just as Fouriers law and the heat balance can be employed to characterize temperature distribution, analogous relationships are available to model field problems in other areas of engineering. For...
-
(a) Explain why the concentration of dissolved oxygen in freshwater is an important indicator of the quality of the water. (b) How is the solubility of oxygen in water affected by increasing...
-
The reaction catalyzed by malate dehydrogenase, has a G°' = 4.6 kJ mol-1. (a) Would this reaction occur spontaneously in a cell? (b) How does the citrate synthase reaction promote the malate...
-
Predict whether creatine kinase will operate in the direction of ATP synthesis or phosphocreatine synthesis at 25C when [ATP] = 4 mM, [ADP] = 0.15 mM, [phosphocreatine] = 2.5 mM, and [creatine] = 1...
-
If intracellular [ATP] = 5 mM, [ADP] = 0.5 mM, and [Pi] = 1.0 mM, calculate the concentration of AMP at pH 7 and 25C under the condition that the adenylate kinase reaction is at equilibrium.
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...

Study smarter with the SolutionInn App


