Obtain a cubic equation in k 2 , from (5.50), and analyze the dispersion relations for these
Question:
Obtain a cubic equation in k2 , from (5.50), and analyze the dispersion relations for these three modes of wave propagation across the magnetic field in a fully ionized warm plasma.
Equation
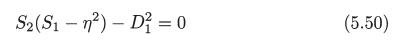
Transcribed Image Text:
S2(S1n²) D² = 0 (5.50)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Equation 550 is given as SS D 0 Here S S and D are parameters related to wave propagation in a fully ...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Another type of wave mode that can be found from a fluid description of a plasma (but requires a kinetic treatment to understand completely) is a drift wave. Just as the two-stream instability...
-
Show that the reflection points w 01 and w 02 , for the LCP and RCP waves propagating along the magnetic field in a fully ionized warm plasma (see Fig. 9) are given, respectively, by Compare these...
-
From the dispersion relations obtained in problem 16.8 show that, in the limit of ci , we obtain the dispersion relation for the (shear) Alfven wave (cold plasma limit of the magnetosonic wave)...
-
ZA Berhad is an investment holding company located in Malaysia. Property development, building, property investment, and management services are the primary activities of the corporation and its...
-
Company A's total capital consists of $150 million in debt, $50 million in leased assets, no outstanding preferred stock, $500 million in common stock, and $300 million in retained earnings. Its...
-
As the financial manager of an unlisted manufacturing company based in Amsterdam, you have been tasked with preparing your firm for potential listing on Euronext. The company is closely held with...
-
The price of gasoline. The yearly average price of unleaded regular gasoline has fluctuated as follows: 1987: $0.95 per gallon 1997: $1.23 per gallon 2007: $2.80 per gallon Give the gasoline price...
-
At December 31, MediSharp Precision Instruments owes $50,000 on accounts payable, salary payable of $16,000, and income tax payable of $8,000. MediSharp also has $280,000 of bonds payable that were...
-
E 3 . 1 9 ( LO 4 ) ( Allocate Transaction Price ) Shaw Company sells goods that cost $ 3 0 0 , 0 0 0 to Ricard Company for $ 4 1 0 , 0 0 0 on January 2 , 2 0 2 5 . The sales price includes an...
-
Starting from (5.12), (5.40), (5.41), and (5.42), provide all the necessary steps to obtain (5.43). Equations C.E=0 (5.12)
-
Make plots analogous to Figs. 9, 10, and 11 for wave propagation in a fully ionized warm plasma, but in terms of as a function of the real part of k. Figure 9 Figure 10. Figure 11. Vph4 U Vse V V sp...
-
Prove that the determinant of each leading submatrix of a symmetric positive definite matrix is positive.
-
How much Group Revenue do you currently have booked for September 2024? (format $, no decimals; e.g, $5,000) How much additional Group Revenue do you need to book to hit your budget for September...
-
What strategies do businesses have in place that promote equal opportunity within the organization? Do most businesses offer career development and training to its employees? If so, why? How do...
-
How do you do the step method in cost accounting? I need help understanding the difference between the step method and reciprocal method.
-
1- How do long-term care changing the health care system in the 21st century? What ethical issues do you see playing a role in this situation? 2- Why do long-term health care and palliative care...
-
How do the processes and layouts of Harley Davidson enable it to deliver goods and services to its customer? (How do they do what they do with the resources they have?)
-
Ember Company purchased a building with a market value of $280,000 and land with a market value of $55,000 on January 1, 2018. Ember Company paid $15,000 cash and signed a 25-year, 12% mortgage...
-
Evaluate each logarithm to four decimal places. log 0.257
-
Based on your answer to Problem 17.67, propose a mechanism for the following transformation: Answer Problem 17.67 Heat CO2 heat
-
Compare the structures of 1,4-pentadiene and divinyl amine: The first compound does not absorb UV light in the region between 200 and 400 nm. The second compound does absorb light above 200 nm. Using...
-
Provide a systematic name for each of the following compounds. a. b. c. d. e. H. CH3 Br
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...

Study smarter with the SolutionInn App


