The entropy of a system can be expressed in terms of the distribution function as Prove that,
Question:
The entropy of a system can be expressed in terms of the distribution function as
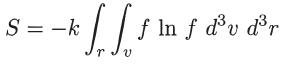
Prove that, for a Maxwellian distribution function, the entropy satisfies the following thermodynamic relations:
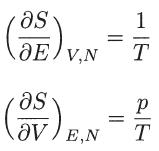
where N is the total number of particles in the system, V is the total volume, and E = 3NkT/2 is the total energy.
Transcribed Image Text:
S = -k [ [ f m f d³v d³ r ln r
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To prove the thermodynamic relations involving entropy for a Maxwellian distribution function lets start with the expression for entropy S in terms of ...View the full answer

Answered By

Anjali Arora
Having the experience of 16 years in providing the best solutions with a proven track record of technical contribution and appreciated for leadership in enhancing team productivity, deliverable quality, and customer satisfaction. Expertise in providing the solution in Computer Science, Management, Accounting, English, Statistics, and Maths.
Also, do website designing and Programming.
Having 7 yrs of Project Management experience.
100% satisfactory answers.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In this problem, you will calculate the transmission probability through the barrier illustrated in Figure 16.10. We first go through the mathematics leading to the solution. You will then carry out...
-
Exercise 5.5 explored the enthalpy representation of thermodynamics for an equilibrium ensemble of systems in contact with a volume bath. Here we extend that analysis to an ensemble out of...
-
The entropy of a system can be expressed, in terms of the distribution function, as Show that, for a system that obeys the collisionless Boltzmann equation, the total time derivative of the entropy...
-
Pick a company or brand or business on which to focus. What business is it inP Who are its direct and indirect competitors? Which in each category are the most relevant competitors?
-
Why are both the price elasticity of demand and the price elasticity of supply likely to be greater in the long run?
-
a. What is the purpose of bond seniority provisions? b. After a call protection period has elapsed, why is the call price an effective ceiling on the market price of a callable bond with a...
-
The placebo effect. A survey of physicians found that some doctors give a placebo to a patient who complains of pain for which the physician can find no cause. If the patients pain improves, these...
-
American Investor Group is opening an office in Portland, Oregon. Fixed monthly costs are office rent ($8,000), depreciation on office furniture ($1,800), utilities ($2,200), special telephone lines...
-
Statement of Cash Flows (Indirect Method) The Rainbow Company's income statement and comparative balance sheets as of December 31 1 of 2013 and 2012 follow: RAINBOW COMPANY Income Statement For the...
-
The distribution of thermal kinetic energies E, for a gas in the Maxwellian state, is given by Calculate the most probable energy and show that the velocity of the particles, which have this energy,...
-
Derive an expression for the Doppler intensity profile (thermal broadening) of a spectral line emitted near the central frequency 0 , assuming that the emitting atoms have a Maxwellian velocity...
-
When is it appropriate to use stratified random sampling? What are strata, and how should strata be selected?
-
Assume an organic compound has a partition coefficient between water and ethyl acetate equal to 8.12. If there are initially 7.10 grams of the compound dissolved in 75.0 mL of water, how many grams...
-
Berger Paint Pakistan limited produces three types of joint products, S ilver paint, G olden paint and D iamond paint. During March, 2020, the following information was recorded : Particulars Silver...
-
Choose an industry in which you are interested in working. How is that industry trending? What internal and external factors may affect the direction of the organization? For your initial post,...
-
show all work and Computations and if favorable or unfavorable. Standard Cost Data per 1 Unit Quantity Price Direct Labor 2 hrs $4.00/hr Actual Data: Units produced 20 20 Direct Labor 30 hrs; total...
-
4. A petroleum company is considering the expansion of its one unloading facility at its Australian refinery. Due to random variations in weather, loading delays, and other factors, ships arriving at...
-
Process costing: university enrolments Flagstaff University is exploring the possibility of outsourcing part or all of its enrolment function. This function involves three separate processes: (a)...
-
Perform the operation by first converting the numerator and denominator to scientific notation. Write the answer in scientific notation. 7,200,00/0.000009
-
Write an equation for the proton transfer reaction that occurs when each of the following bases reacts with water. In each case, draw curved arrows that show the mechanism of the proton transfer: (a)...
-
If we compare the sizes of the halogens, we find that they increase in size from fluorine to iodine. Nevertheless, fluoroethane, chloroethane, bromoethane, and iodoethane all have very similar...
-
Identify the number of stereoisomers expected for each of the following: a. b. c. d. e. f. CI
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


