A flow of 1 kg/s carbon dioxide at 1600 K, 100 kPa is mixed with a flow
Question:
A flow of 1 kg/s carbon dioxide at 1600 K, 100 kPa is mixed with a flow of 2 kg/s water at 800 K, 100 kPa, and after the mixing it goes through a heat exchanger, where it is cooled to 500 K by a 400 K ambient. How much heat transfer is taken out in the heat exchanger? What is the entropy generation rate for the whole process?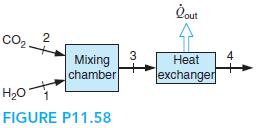
Transcribed Image Text:
Qut Co2 2 3 4 Mixing chamber Heat exchanger H20 FIGURE P11.58
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 92% (13 reviews)
The amount of heat taken out in the heat exchanger will be 1 kgs x 1600 K 2 kgs x 800 K 3 kgs x 5...View the full answer

Answered By

Firoz K
I have extensive experience in education and tutoring, having worked as a tutor for the past three years in both group and individual settings. During my time as a tutor, I have successfully helped students improve their academic performance in a variety of subjects, including mathematics, science, language arts, and social studies. I have also developed and implemented personalized learning plans and differentiated instruction techniques to accommodate the individual needs of my students. Moreover, I have effectively communicated with parents and teachers to ensure that the students receive the best possible education and guidance. My strong organizational, communication, and problem-solving skills have enabled me to successfully collaborate with students, parents, and teachers in order to provide an effective and enjoyable learning experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
A flow of 1 kg/s carbon dioxide at 1600 H, 100 kPa is mixed with a flow of 2 kg/s water at 800 H, 100 kPa and after the mixing it goes through a heat exchanger where it is cooled to 500 H by a 400 H...
-
A reversible steady state device receives a flow of 1 kg/s air at 400 K, 450 kPa and the air leaves at 600 K, 100 kPa. Heat transfer of 800 kW is added from a 1000 K reservoir, 100 kW rejected at 350...
-
A flow of 1 kg/s saturated moist air (relative humidity 100%) at 100 kPa, 10oC goes through a heat exchanger and comes out at 25oC. What is the exit relative humidity and how much power is needed?
-
Why is it likely to have preexisting normal faults in an orogenic belt?
-
Apply the rank order clustering technique to the part-machine incidence matrix in the following table to identify logical part families and machine groups. Parts are identified by letters, and...
-
Two couples act on the frame. Determine the resultant couple moment. Compute the result by resolving each force into x and y components and (a) Finding the moment of each couple (Eq. 4 -13) and (b)...
-
Malicious attacks on a cryptocurrency network. Refer to the Computers & Security (January 2020) study of a software system developed for cryptocurrency, Exercise
-
1. K2 Network operates online game sites used by about 16 million people in over 100 countries. Players are allowed to enter a game for free, but must buy digital assets from K2, such as swords to...
-
These calculations are repeated for every month of the project. Cash Flow Statement Bought Forward January February March April May June $5,000 ncome $10,000 Total $15,000 Available Expenses: $300...
-
Russell Engineering provides consulting services related to land development. Below is the year-end adjusted trial balance of Russell Engineering. Accounts Cash Accounts Receivable Supplies Prepaid...
-
A mixture of 60% helium and 40% nitrogen by mass enters a turbine at 1 MPa, 800 K at a rate of 2 kg/s. The adiabatic turbine has an exit pressure of 100 kPa and an isentropic efficiency of 85%. Find...
-
A flow of 2 kg/s mixture of 50% carbon dioxide and 50% oxygen by mass is heated in a constant pressure heat exchanger from 400 K to 1000 K by a radiation source at 1400 K. Find the rate of heat...
-
Prepare a five-minute presentation, including graphics, on one of the topics listed here. For each presentation, your audience will consist of the other students in your class and your purpose is to...
-
The State Public Works Division consists of the Administrator, the State Public Works Board, the Public Works Section, and the Buildings and Grounds Section. The State Public Works Board consists of...
-
Everyone knows that health care costs are high. It is also known that people tend to spend less on health care if they spend their own money, which motivated the creation of flexible spending...
-
Write a essay that critically evaluates issues in financing health care by addressing the provided prompts. Educate operational leadership on why it is important to the overall bottom line of the...
-
What are the types of conflicts that individuals may have at work? Which type have you experienced the most? 2. What are some primary causes of conflict at work? 3. Explain how miscommunication might...
-
What program do you work with that has a budget? Navy JROTC Who helps to determine how the funds are allocated and spent? US Navy and St. Elizabeth ISD How did you find out the budget amount? Does...
-
Write a program that draws line segments using the arrow keys. The line starts from the center of the pane and draws toward east, north, west, or south when the right-arrow key, up-arrow key,...
-
9.Consider the reaction 3NO2(g)+H2O=2HNO3(aq)+NO(g) where Delta H=-137 kJ.How many kilojoules are released when 92.3g of NO2 reacts?
-
How many signals would you expect in the 13 C NMR spectrum of each of the compounds in Problem 16.34? In Problem 16.34 How many signals would you expect in the 1 H NMR spectrum of each of the...
-
How would you distinguish between the following compounds using 13 C NMR spectroscopy?
-
Predict the multiplicity of each signal in the 1 H NMR spectrum of the following compound:
-
Jennifer purchased a home for $1,000,000 in 2016. She paid $200,000 cash and borrowed the remaining $800,000. This is Jennifer's only residence. Assume that in year 2024, when the home had...
-
business plan describing company with strengths and weaknesses. Any gaps in plan. Recommendations for improvement of the plan.
-
You wish to buy a car today for $35,000. You plan to put 10% down and finance the rest at 5.20% p.a. for six years. You will make equal monthly payments of $_______.

Study smarter with the SolutionInn App


