A refrigerator using R-410a is powered by a small natural gas-fired heat engine with a thermal efficiency
Question:
A refrigerator using R-410a is powered by a small natural gas-fired heat engine with a thermal efficiency of 25%, as shown in Fig. P9.110. The R-410a condenses at 40◦C and evaporates at −20◦C, and the cycle is standard. Find the two specific heat transfers in the refrigeration cycle. What is the overall COP as QL/Q1?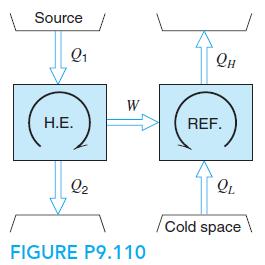
Transcribed Image Text:
Source Q1 Он W Н.Е. REF. Q2 Cold space FIGURE P9.110
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
High temperature 1000K Compressor Work 107 kJkg Turbine Work 162 kJkg The ideal Ericsson cycle consi...View the full answer

Answered By

Firoz K
I have extensive experience in education and tutoring, having worked as a tutor for the past three years in both group and individual settings. During my time as a tutor, I have successfully helped students improve their academic performance in a variety of subjects, including mathematics, science, language arts, and social studies. I have also developed and implemented personalized learning plans and differentiated instruction techniques to accommodate the individual needs of my students. Moreover, I have effectively communicated with parents and teachers to ensure that the students receive the best possible education and guidance. My strong organizational, communication, and problem-solving skills have enabled me to successfully collaborate with students, parents, and teachers in order to provide an effective and enjoyable learning experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
A refrigerator using R-22 is powered by a small natural gas fired heat engine with a thermal efficiency of 25%. The R-22 condenses at 40C and it evaporates at-20C and the cycle is...
-
A Carnot heat engine with a thermal efficiency of 60% receives heat from a source at a rate of 3000 kJ/min, and rejects the waste heat to a medium at 300 K. Determine (a) The power that is generated...
-
A heat engine with a thermal efficiency of does 500 J of net work each cycle. How much heat per cycle is lost to the low-temperature reservoir?
-
Olena Mirrors records bad debt using the allowance, income statement method. They recorded $343,160 in accounts receivable for the year and $577,930 in credit sales. The uncollectible percentage is...
-
A unit load AS/RS for work-in-process storage in a factory must be designed to store 2000 pallet loads, with an allowance of no less than 20% additional storage compartments for peak periods and...
-
A capacitor of capacitance C1 = 1.0F carrying initially a voltage V = 300 V is connected in parallel with an uncharged capacitor of capacitance C2: = 2.0F. Find the increment of the electric energy...
-
Coupon user study. A hot topic in marketing research is the exploration of a technology-based self-service (TBSS) encounter, e.g., ATMs, automated hotel checkout, online banking, and express package...
-
Included in Gonzalez Companys December 31 trial balance is a note receivable of $12,000. The note is a 4-month, 10% note dated October 1. Prepare Gonzalezs December 31 adjusting entry to record $300...
-
View Policies Current Attempt in Progress From the following, identify if the transaction results in an understatement or overstatement of net income reported on the income statement for the year...
-
John Wallace is an automotive enthusiast. He has over 25 years of experience as a mechanic for the dealership of a large car manufacturer in Oakville. John also gained experience doing minor body...
-
As explained in the previous problem, the ammonia absorption cycle is very similar to the setup sketched in Problem 9.110. Assume the heat engine has an efficiency of 30% and the COP of the...
-
A split evaporator is used to provide cooling of the refrigerator section and separate cooling of the freezer section, as shown in Fig. P9.109. Assume constant pressure in the two evaporators. How...
-
Tien is a citizen of Country C, which does not have an income tax treaty with the United States. During the current year (2016), she is a nonresident alien for U.S. tax purposes and earns the...
-
how is lateral force(fy) determined from this data Tyre Responses 1 1 1 1 1 1.3 1.3 1.3 1.3 1.3 1.6 1.55 1.45 1.27 1.1 Fz (N) 0 400 800 1200 1500 Slip Angle (deg) Fy1 (N) Fy2 (N) Fy3 (N) 0.0 0 0 0.5...
-
(13%) Problem 8: A wire is oscillated to create a wave of the form y(x,t) = Asin(x - 30t) == The wave is reflected from a fixed end producing a reflection of the form y2(x,t) = A sin(x + 30t) The two...
-
Using the definitions of even integer and odd integer, give a proof by contraposition that this statement is true for all integers n: If 5n+3 is even, then n is odd.
-
7. Design the formwork for a wall 8-ft (2.44-m) high to be poured at the rate of 5 ft/h (1.53 m/h) at a temperature of 77F (25C). The concrete mixture will use Type I cement without retarders and is...
-
tempt in Progress The City of Minden entered into the following transactions during the year 2026. 1. A bond issue was authorized by vote to provide funds for the construction of a new municipal...
-
A CPA who is a covered person purchased stock in a client corporation and placed it in a trust as an educational fund for the CPAs minor child. The trust securities were not material to the CPA but...
-
Explain five different cases of income exempt from tax with clear examples.
-
Draw the structure of each possible dichloride that can be used to prepare the following alkyne via elimination:
-
Draw the structures of compounds A to D: NaNH, NaNH, 1) Excess NaNH, Br2 Br2 2) H20 (C3H12)
-
Consider the equilibrium 3O 2 (g) 2O 3 (g). a. Using the Data tables, calculate K P at 298 K. b. Assuming that the extent of reaction at equilibrium eq is much less than 1, show that the degree of...
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


