Consider the combination of the two heat engines, as in Fig. P5.4. How should the intermediate temperature
Question:
Consider the combination of the two heat engines, as in Fig. P5.4. How should the intermediate temperature be selected so that the two heat engines have the same efficiency, assuming Carnot cycle heat engines.
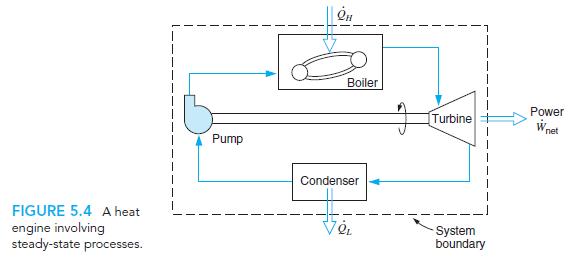
Transcribed Image Text:
Boiler Power Turbine W net Pump Condenser FIGURE 5.4 A heat engine involving steady-state processes. System boundary
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
The intermediate temperature should be selected so that ...View the full answer

Answered By

Firoz K
I have extensive experience in education and tutoring, having worked as a tutor for the past three years in both group and individual settings. During my time as a tutor, I have successfully helped students improve their academic performance in a variety of subjects, including mathematics, science, language arts, and social studies. I have also developed and implemented personalized learning plans and differentiated instruction techniques to accommodate the individual needs of my students. Moreover, I have effectively communicated with parents and teachers to ensure that the students receive the best possible education and guidance. My strong organizational, communication, and problem-solving skills have enabled me to successfully collaborate with students, parents, and teachers in order to provide an effective and enjoyable learning experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Consider the combination of capacitors in figure.(a) What is the equivalent capacitance of the group?(b) Determine the charge on eachcapacitor. 24.0 F 2.00 F 4.00 F 36.0 V 8.00 uf
-
Consider the combination of a heat engine and a heat pump, as given in Problem 5.41, with a low temperature of 720 R. What should the high temperature be so that the heat engine is reversible? For...
-
Consider two Carnot heat engines operating in series. The first engine receives heat from the reservoir at 1800 K and rejects the waste heat to another reservoir at temperature T. The second engine...
-
In figure, one end of a uniform beam weighing 20 x V3N is attached to a wall with a hinge. The other end is supported by a wire connected to the wall as shown. If the tension in the wire is a x 10N,...
-
What is the value of the mean score for any standard normal distribution? What is the value of the standard deviation for any standard normal distribution? Explain why this is true for any standard...
-
What particle (a particle, electron, or positron) is emitted in the following radioactive decays? (a) 2714Si 2713Al; (b) 23892U 23490Th; (c) 7433As 7434Se.
-
Explain how a derivative asset can act as insurance for losses from the spot price of a financial asset or commodity changing.
-
Simulate Problem 28 to find the probabilities of project completion. Use a triangular distribution for defining activity time distributions. After a 1,000-trial simulation, examine the statistical...
-
Given the following partial amortization table for a bond with a face value of $500.00, paying a semi-annual coupon, and priced to yield 2.250% compounded monthly: Time K(t) I(t) Amort. of...
-
You are in the market for a new car. You do not have a trade-in, but you have saved $2,500 toward a down payment. You currently earn $4,000.00 gross monthly income, of which 35% is withheld for...
-
The air conditioner in the previous problem is turned off. How quickly does the house heat up in degrees per second (C/s)? Data from previous problem An air conditioner on a hot summer day removes 8...
-
Consider a combination of a gas turbine power plant and a steam power plant, as shown in Fig. P5.4. The gas turbine operates at higher temperatures (thus called a topping cycle) than the steam power...
-
Describe how management practices and organizational behaviour can help organizations deal with the contemporary management concerns discussed in the chapter. In other words, what are some of the...
-
Adidas-Consumer Goods STEP ONE: MISSION: Mission statement core message that guides and influences your marketing strategy. Why is this company in business and what is the purpose of their...
-
You have been operating and growing your golf club for the last six (6) years. You are happy with the fact that all revenue streams (and as a result your share value) have continued to increase as...
-
Given the following HTML, write a simple bit of JavaScript code that will DELETE ALL OF THE TAGS ON THE PAGE. Quiz I'm a Heading I'm a paragraph I'm special I'm also a paragraph Footer! HINT: You'll...
-
Your company has been quite successful in sending employees on international assignments. As the HR Manager responsible for selecting such employees, present a report to the management of your...
-
You will be looking at a particular market in the economy. I will assign the market to you arbitrarily. Please look for at the end of this document to identify which market you will be responsible...
-
You have just started a sales job in a department store. Your pay consists of a base salary and a commission. The base salary is $5,000. The scheme shown below is used to determine the commission...
-
United Business Forms capital structure is as follows: Debt ............................................ 35% Preferred stock ........................... 15 Common equity .......................... 50...
-
In each case, identify the most likely position at which monobromination would occur. (a) (b) (c) (d) N.
-
Identify the carboxylic acid and the alcohol that are necessary in order to make each of the following compounds via a Fischer esterification: a. b. c. CH 3 CH 2 CO 2 C (CH 3 ) 3
-
Determine the structures of compounds A through F: Na,Cr,0, H2SO4, H20 [H'] A EtO soch 1) LIAI(OR)H `2) H20 xS NH3
-
Deacon Company is a merchandising company that is preparing a budget for the three - month period ended June 3 0 th . The following information is available Deacon Company Balance Sheet March 3 1...
-
Mango Company applies overhead based on direct labor costs. For the current year, Mango Company estimated total overhead costs to be $460,000, and direct labor costs to be $230,000. Actual overhead...
-
Which of the following do we expect to be the horizon growth rate for a company (long term growth rate- say 30-50 years)? A) Inflation B) Industry Average C) Zero D) Market Beta

Study smarter with the SolutionInn App


