CV A is the mass inside a piston/cylinder; C V B is that plus part of the
Question:
CV A is the mass inside a piston/cylinder; CV B is that plus part of the wall out to a source of 1Q2 at Ts. Write the entropy equation for the two control volumes, assuming no change of state of the piston mass or walls.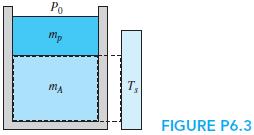
Transcribed Image Text:
Po mp T, FIGURE P6.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 92% (13 reviews)
CV A SA mACvlnTATA0 CV B SB mACvlnTBTA0 mBCvlnTBTB0 The entropy equation ...View the full answer

Answered By

Firoz K
I have extensive experience in education and tutoring, having worked as a tutor for the past three years in both group and individual settings. During my time as a tutor, I have successfully helped students improve their academic performance in a variety of subjects, including mathematics, science, language arts, and social studies. I have also developed and implemented personalized learning plans and differentiated instruction techniques to accommodate the individual needs of my students. Moreover, I have effectively communicated with parents and teachers to ensure that the students receive the best possible education and guidance. My strong organizational, communication, and problem-solving skills have enabled me to successfully collaborate with students, parents, and teachers in order to provide an effective and enjoyable learning experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
In Fig. P3.5, CV A is the mass inside a piston/ cylinder, and CV B is the mass plus the piston outside, which is the standard atmosphere. Write the energy equation and work term for the two CVs,...
-
A piston can freely move inside a horizontal cylinder closed from both ends. Initially, the piston separates the inside space of the cylinder into two equal parts each of volume V0, in which an ideal...
-
A piston - cylinder device initially contains 0.35-kg steam at 3.5 MPa, superheated by 7.4oC. Now the steam loses heat to the surroundings and the piston moves down, hitting a set of stops at which...
-
Consider the circuit shown in (Figure 1). Suppose that v 240 V,v 2 110 V, and v 3 450 V Figure 1 of 1 a) Use the mesh-current method to find the magnitude of the total power developed in the circuit...
-
Infant mortality rates are a key indicator of the quality of health care a country can provide for its citizens. The following table shows infant mortality rates for selected countries based on...
-
A uniform slender L-shaped bar ABC is at rest on a horizontal surface when a force P of magnitude 4 N is applied at point A. Neglecting friction between the bar and the surface and knowing that the...
-
The following are the financial statements of Persona Ltd for last year and this year: Calculate the following ratios for both years: Return on net assets Return on equity Gross profit margin Net...
-
The City of Rochester signed a 30-year agreement with East Coast Real Estate, Inc. to lease a newly constructed building for city services. The city agrees to make an initial payment of $1,000,000...
-
answer fastly 2 5 1 .
-
Lou and Joann Girardi are married and file a joint return. They recently bought a new home on 21680 Skyline Drive, Henderson, NV 89077. Their son, Stuart, attends the University of Pennsylvania...
-
A window air conditioner cools a room at T L = 68 F with a maximum of 1.2 kW power input. The room gains 0.33 Btu/s per degree temperature difference from the ambient, and the refrigeration COP is =...
-
Consider the previous setup with the mass m A and the piston/cylinder of mass m p starting out at two different temperatures (Fig. P6.3). After a while, the temperature becomes uniform without any...
-
A 20.0 L stainless steel container at 25 o C was charged with 2.00 atm of hydrogen gas and 3.00 atm of oxygen gas. A spark ignited the mixture, producing water. What is the pressure in the tank at 25...
-
Fineas Co. use the Job Order Costing system to determine product costs. Before entering 2020, the company has created a production budget, with an estimated total manufacturing overhead of $...
-
Define what a market value is? What are three major principles of investing funds? How does the federal government control the money supply? An investor purchases a 10-year U.S. Treasury note and...
-
1. Suppose we have two alternative designs, each of which yields a different present value of the total lifetime cost: the first is $1604 and the second is $1595. Verify that the present value of the...
-
Sometimes when we are asked for a linear model, the information that we are given is data about a scenario. In these cases we have to use Excel to generate a trendline. There is a video in this...
-
1. Purpose Explain 3 points from the Introduction section as to why this study is important. How did this study build on the existing literature in this area? 2. Participants Outline at least 2...
-
Write a method that converts milliseconds to hours, minutes, and seconds using the following header: public static String convertMillis(long millis) The method returns a string as...
-
How has the too-big-to-fail policy been limited in the FDICIA legislation? How might limiting the too-big-to-fail policy help reduce the risk of a future banking crisis?
-
Calculate the vapor pressure of CH 3 OH(l) at 298.15 K if He is added to the gas phase at a partial pressure of 200. Bar using the data tables. By what factor does the vapor pressure change?
-
Use the vapor pressures of SO 2 (l) given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. T (K) 190. P (Pa) T (K) 230. P...
-
Prove that a substance for which the solidliquid coexistence curve has a negative slope contracts upon melting.
-
Vaughn Company sells two types of pumps. One is large and is for commercial use. The other is smaller and is used in residential swimming pools. The following inventory data is available for the...
-
To fund your dream around-the-world vacation, you plan to save $1,300 per year for the next 14 years starting one year from now. If you can earn an interest rate of 5.83 percent, how much will you...
-
On NSE (Indian stock exchange), shares of ICICI Bank trade for 935 rupees. If the spot exchange rate is USD 0.012, what is the no-arbitrage USD price of ICICI Bank ADR? Assume that transactions costs...

Study smarter with the SolutionInn App


