Consider the previous setup with the mass m A and the piston/cylinder of mass m p starting
Question:
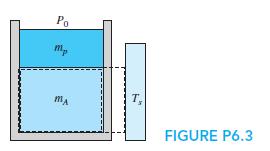
Consider the previous setup with the mass mA and the piston/cylinder of mass mp starting out at two different temperatures (Fig. P6.3). After a while, the temperature becomes uniform without any external heat transfer. Write the entropy equation storage term (S2 − S1) for the total mass.
In figure P6.3
Transcribed Image Text:
Po T, FIGURE P6.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
The entropy equation for the storage term S2 S1 f...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
In Fig two isotropic point sources S1 and S2 emit identical light waves in phase at wavelength . The sources lie at separation d on an x axis, and a light detector is moved in a circle of large...
-
In Figure two isotropic point sources S1 and S2 emit light at wavelength ? = 400 nm. Source S1 is located at y = 640 nm; source S2 is located at y = ?? 640 nm. At point P1 (at x = 120 nm), the wave...
-
Figure shows two point sources, S1 and S2 that emit light at wavelength = 500 nm and with the same amplitude. The emissions are isotropic and in phase, and the separation between the sources ts d =...
-
Ivanhoe Company was formed on July 1, 2018 It was authorized to issue 285,500 shares of $10 par value common stock and 95,400 shares of 8 $25 par value, cumulative and nonparticipating preferred...
-
Many policymakers are interested in different measures of participation in the labor force. Use the information from the 2010 GSS to explore how labor force participation varies by sex (not all...
-
A drum of 200-mm radius is attached to a disk of radius rA = 150 mm. The disk and drum have a combined mass of 5 kg and a combined radius of gyration of 120 mm and are suspended by two cords. Knowing...
-
The following are the highly simplified financial statements of Duration Ltd for last year: Calculate as many accounting ratios as the information provided will allow. Turnover Profit and loss...
-
Standard Bent Glass wanted to buy a machine for its factory that would produce cut glass. In March 1998, it started negotiations with Glassrobots Oy, a Finnish corporation. By February 1999,...
-
Whartons changed to a _______ benefit plan in order to allow employees to choose the plan they preferred. Multiple Choice cafeteria-style forced standard fixed inflexible
-
After watching the Belgian / French film "Fear and Trembling" - [rental link - Rental link to the Belgian / Japanese film Prompt 1: Imagine that you took over Amelie's position in that company after...
-
CV A is the mass inside a piston/cylinder; C V B is that plus part of the wall out to a source of 1Q 2 at T s . Write the entropy equation for the two control volumes, assuming no change of state of...
-
Water at 100C, quality 50% in a rigid box is heated to 110C. How do the properties (P, v, x, u, and s) change (increase, stay about the same, or decrease)?
-
Discuss why financial analysts consider antiques and art to be illiquid investments. Why do they consider coins and stamps to be more liquid than antiques and art? What must an investor typically do...
-
In this Capstone experience, you will develop a strategy playbook for a selected organization. You may be familiar with the concept of a playbook as it relates to a sports team, but what might that...
-
On January 1, 2024, the general ledger of Big Blast Fireworks includes the following account balances: Accounts Cash Debit Credit $25,900 Accounts Receivable 46,500 Allowance for Uncollectible...
-
The WRX can travel 1 / 4 of a mile in 1 3 . 9 sec . Calculate the acceleration over this distance if assumed constant.
-
You are expected to develop two simulators mimicking the behavior and analyze the performance of iterative multiplication algorithm and add-and-shift multiplication algorithm. You are free to use any...
-
A.BRK Company, which Manufactures bags, has a Capacity of 130,000 bags per month. Currently its operating capacity is 100,000 units. The company receives a special order of 20,000 bags at $9 a bag. A...
-
A palindromic prime is a prime number and also palindromic. For example, 131 is a prime and also a palindromic prime, as are 313 and 757. Write a program that displays the first 100 palindromic prime...
-
Are the two PT phase diagrams below likely to be observed for a pure substance? If not, explain all features of the diagram that will not be observed. a. b. Liquid Vapor Solid Solid II Liquid Solid...
-
The vapor pressure of methanol(l) is 16.94 10 3 Pa at 298.15 K. Use this value to calculate G o f (CH 3 OH, g) G o f (CH 3 OH, l). Compare your result with those in Table 4.1.
-
Within what range can you restrict the values of P and/or T if the following information is known about sulfur? Use Figure 8.11 to answer this problem. a. Only the rhombic solid phase is observed for...
-
There is a credit rating agency for businesses that gives out various amounts of information based on the subscription level. This company is called a. Business Credit Scoring b. Fair Issue c. Dun...
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...

Study smarter with the SolutionInn App


