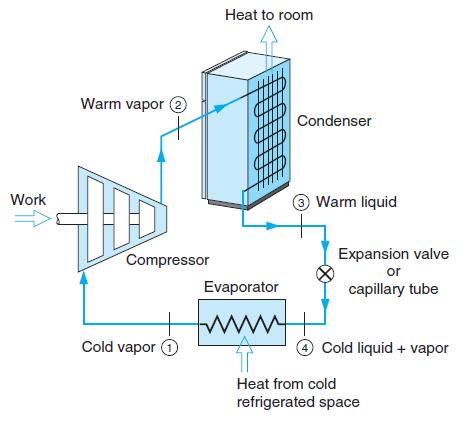
Make a control volume around the refrigerator in Fig. 1.3. Identify the mass flow of external air
Question:
Make a control volume around the refrigerator in Fig. 1.3. Identify the mass flow of external air and show where you have significant heat transfer and where storage changes.
Transcribed Image Text:
Heat to room Warm vapor e Condenser Work Warm liquid Expansion valve or capillary tube Compressor Evaporator Cold vapor O O Cold liquid + vapor Heat from cold refrigerated space
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (18 reviews)
mass flowrate m 1 Compressor work W c mh2 ...View the full answer

Answered By

Hardik Dudhat
I am semi-qualified Chemical Engineering ,I have scored centum in accounting in my senior secondary and in my graduation. I have always helped my fellow students with their concerns on the subject, i have tutored on various tutoring sites in the past and also have taken home tuitions for degree and MBA students. As a tutor, I don't want my students to just get a solution, I want them to understand the concept and never have a doubt in that area thereon and i believe in excelling and not in educating.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Make a control volume around the whole power plant in Figure 1.2 and with the help of Fig. 1.1 list what flows of mass and energy are in or out and any storage of energy. Make sure you know what is...
-
Make a control volume around the whole power plant in Figure 1.2 and with the help of Fig. 1.1 list what flows of mass and energy are in or out and any storage of energy. Make Sure you know what is...
-
Make a control volume around the turbine in the steam power plant in Fig. 1.1 and list the flows of mass and energy that are there.
-
47GP: Chapter: CH0 CH1 CH2 CH3 CH4 CH5 CH6 CH7 CH8 CH9 CH10 CH11 CH12 CH13 CH14 CH15 CH16 CH17 CH18 CH19 CH20 CH21 CH22 CH23 CH24 CH25 CH26 CH27 CH28 CH29 CH30 Problem: 1CQ 1MCP 1P 2CQ 2MCP 2P 3CQ...
-
Based on the MTF11SSD, examine the relationship between a teen's race (RACE) and the number of friends who drink alcohol (FRDRINK) and smoke cigarettes (FRSMOKE). Using SPSS Crosstabs, create two...
-
A 750-g collar can slide along the horizontal rod shown. It is attached to an elastic cord with an undeformed length of 300 mm and a spring constant of 150 N/m. Knowing that the collar is released...
-
In an effort to reduce customer dissatisfaction with delays in replacing lost automated teller machine (ATM) cards, some retail banks monitor the time required to replace a lost ATM card. Called...
-
Listed are the equity sections of balance sheets for years 2011 and 2012 as reported by Mountain Air Ski Resorts, Inc. The overall value of stockholders equity has risen from $2,000,000 to...
-
PROBLEM 3-15 High-Low Method; Predicting Cost [LO1, LO2] Crosshill Company's total overhead costs at various levels of activity are presented below: Total Overhead Cost Month Machine Hours April....
-
1. Name at least three ways that Shu could automate her asset management. Suggest at least one option for retirement savings, general savings, and general convenience. 2. What major factors should...
-
A car rolls down a hill with a slope such that the gravitational pull in the direction of motion is one-tenth of the standard gravitational force (see Problem 1.26). If the car has a mass of 2500 kg,...
-
A piston/cylinder with a cross-sectional area of 0.1 ft 2 has a piston mass of 200 lbm resting on the stops, as shown in Fig. P1.50. With an outside atmospheric pressure of 1 atm, what should the...
-
Record the following transactions in the basic accounting equation. Treat each one separately. Assets = Liabilities + Owners Equity a. Micheal invests $112,000 in company. b. Bought equipment for...
-
Write out the form of the partial fraction decomposition of the function (See Example ). Do not determine the numerical values of the coefficients. (If the partial fraction decomposition does not...
-
Below is the actual assignment information. Here is where you will submit your event for approval. It is not graded, but you will need it to be marked complete in order to submit your paper, so...
-
3. (20 points) A researcher is interested in whether the phonics method of teaching reading is more or less effective than the sight method, depending on what grade the child is in. Twenty children...
-
Let A and B be the matrices given below: -5 9 -7 A= 8 -1 -3 B=9 6 -1 8 -1 -7. 0 Perform the following matrix operations and enter the entries below: -4A = A-4B = 5A-3B=
-
The product business can be isolated into four principal classes: programming administrations, framework administrations, open source and SaaS. The accompanying depicts the classifications of...
-
Write a method to multiply two matrices. The header of the method is:public static double[][]multiplyMatrix(double[][] a,?double[][] b)To multiply matrix a by matrix b, the number of columns in a...
-
Identify Thank You mission, strategy and core competencies. Identify strategy changes that have taken place at Thank You since its founding in 2008. Your answer must in text references and must be...
-
Compare the electrostatic potential maps for cycloheptatrienone and cyclopentadienone. Both of these maps were created using the same color scale so they can be compared. Notice the difference...
-
What is the advantage of a differential scanning calorimeter over a bomb calorimeter in determining the enthalpy of fusion of a series of samples?
-
You wish to measure the heat of solution of NaCl in water. Would the calorimetric technique of choice be at constant pressure or constant volume? Why?
-
Al preparar el estado de resultados pro forma, cules de las siguientes partidas se deducen de las utilidades brutas para llegar a las ganancias despus de impuestos? Pregunta de seleccin mltiple....
-
Lawson Inc. is expanding its manufacturing plant, which requires an investment of $4 million in new equipment and plant modifications. Lawson's sales are expected to increase by $3 million per year...
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...

Study smarter with the SolutionInn App


