Question: (A) Compound A with the formula C 3 H 8 O is soluble in water and reacts with sodium metal, producing bubbles of gas. When
(A) Compound A with the formula C3H8O is soluble in water and reacts with sodium metal, producing bubbles of gas. When compound A is treated with chromic acid (a mixture of Na2Cr2O7 and H2SO4), compound B is formed. Compound B dissolves readily in Na2CO3(aq) and reacts with ethanol, yielding compound C which has a fruity fragrance. Identify compounds A, B, and C.
(B) The following molecules all have the molecular formula C5H10O. You suspect that you have a sample of one of these compounds. What tests could you perform to ascertain which of these compounds you have?
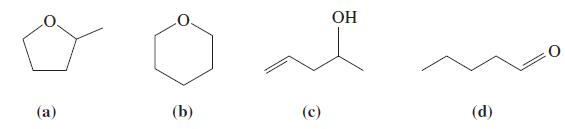
() (b) () HO (d) 0 0
Step by Step Solution
3.30 Rating (156 Votes )
There are 3 Steps involved in it
Question A Compound A Propanol C3H8O Compound B Propanone C3H6O Compound C Ethyl propanoate C5H10O2 Justification Propanol is soluble in water because ... View full answer

Get step-by-step solutions from verified subject matter experts


