When the manometer in Figure 6-5(c) is filled with liquid mercury (d = 13.6 g/cm 3 )
Question:
When the manometer in Figure 6-5(c) is filled with liquid mercury (d = 13.6 g/cm3) the barometric pressure is 748.2 mmHg, and the difference in mercury levels is 8.6 mmHg. What is the gas pressure Pgas?
Figure 6-5(c)
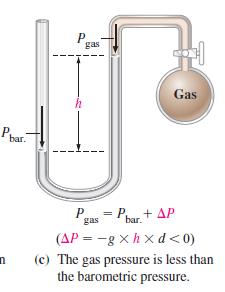
Transcribed Image Text:
Ph bar. n P h gas P gas 220 = Pbar + AP Gas (AP=-gxhxd<0) (c) The gas pressure is less than the barometric pressure.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Analyze We must first establish which is greater the barometric pressure or the ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Suppose that the mercury level in Example 6-2 is 7.8 mm higher in the arm open to the atmosphere than in the closed arm. What would be the value of P gas ? (B) Suppose P bar. and P gas are those...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A cylindrical glass beaker of height 1.520 m rests on a table. The bottom half of the beaker is filled with a gas, and the top half is filled with liquid mercury that is exposed to the atmosphere....
-
Briefly explain the differences between copyrights and patents.
-
Refer to the data and analysis developed for the Magee Company in Exhibits 8.5 and 8.6. Evaluate the alternatives using a cost of capital of 12 percent.
-
Continental had a franchise from GTE to sell its TVs. The franchise agreement stated that Continental could sell GTEs products only from the specific location described in the franchise agreement and...
-
The availability heuristic states that events that are more easily remembered are judged as being more probable than events that are less easily remembered. This heuristic can sometimes lead to...
-
Sound Sensation Inc. is a small manufacturer of electronic musical instruments. The plant manager received the following variable factory overhead report for the period: The plant manager is not...
-
When firms dispose of a long-lived asset by selling it before the end of its useful life, the difference between the net book value of the asset and the disposition proceeds is a/an: Multiple Choice...
-
Calculate the height of a mercury column required to produce a pressure (a) Of 0.984 atm; (b) Of 928 Torr; (c) Equal to that of a column of water 142 ft high.
-
Explain how the action of a water siphon is related to that of a suction pump.
-
A multilayer coil of 2000 turns of fine wire is 20 mm long and has a thickness 5 mm of winding. If the coil carries a current of 5 mA, the mmf generated is (a) 10 A t (b) 500 A t (c) 2000 A t (d)...
-
Bamboo You, Inc. This company manufactures bamboo picture frames that sell for \($20\) each. Each frame requires 4 linear feet of bamboo, which costs \($1.50\) per foot. Each frame takes...
-
Refer to the information in PA9-1. Bamboo You, Inc., had \($9,800\) cash on hand at April 1. Of its sales, 70 percent is in cash. Of the credit sales, 40 percent is collected during the month of the...
-
Debit and Credit Effects Indicate the account that will be credited for each of the following transactions: a. Issued common stock for cash b. Borrowed money from a bank c. Provided services on...
-
Normal Balances Indicate for each of the following accounts whether the normal balance is a debit or a credit: a. Accounts Receivable b. Accounts Payable e. Inventory c. Dividends d. Wage Expense f....
-
Prepare a Trial Balance The following balances were taken from the general ledger of Doogie Corporation as of December 31. All balances are normal. Prepare a trial balance. Cash..... Accounts...
-
If L2 is measured in a hydrogen atom whose state function is that in Prob. 7.32, give the possible outcomes and their probabilities. Then calculate L2 for this state.
-
CLASS PERIO Solving Linear Equations: Variable on Both Sides Solve each equation. 1) 6r+ 7 = 13 + 7r 3) -7x-3x+2=-8x-8 5)-14 +66+7-26=1+5b 7) n-3n = 14-4n 2) 13-4x=1-x 4)-8-x= x - 4x 6)n+2=-14-n 8)...
-
Why didnt the FASB cover both types of postretirement benefitspensions and healthcarein the earlier pension accounting rules?
-
What are the major differences between postretirement healthcare benefits and pension benefits?
-
What is the difference between the APBO and the EPBO? What are the components of postretirement expense?
-
Question 1: In 2015, Mordica Co. issued 200,000 shares of $10 par value ordinary shares at $35 per share. In January, 2016, Mordica repurchased 15,000 shares at $30 per share. Assume these are the...
-
You work in the internal audit department of a company that has an online expense claim system. There are many expenses claims every month, and the audit department can only check a small number due...
-
Crisis de jour! Our director is not cooperating. And, where is the money going? I would like to be able to act rather than react. We must keep the place open! Sarah, chair of the Sunshine Center...

Study smarter with the SolutionInn App


