Determine the shapes of each of the following molecules and then, using the data in Table 2.2,
Question:
Determine the shapes of each of the following molecules and then, using the data in Table 2.2, state whether each is expected to be polar or not:
(a) H2S;
(b) CO2;
(c) SO2;
(d) BF3;
(e) PF5 ;
(f) cis-N2F2;
(g) trans-N2F2;
(h) HCN.
Table 2.2
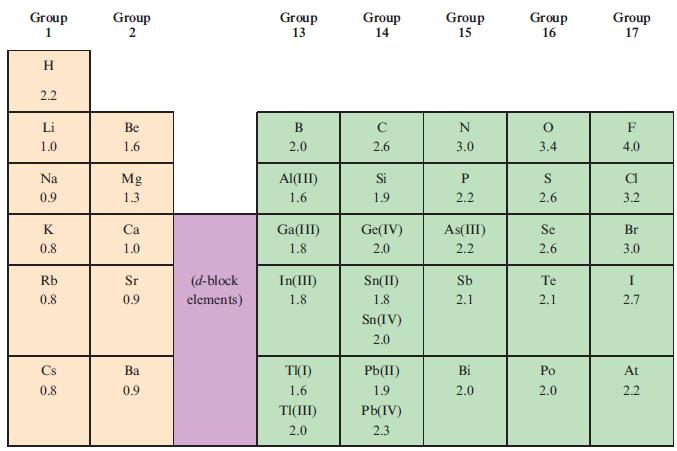
Transcribed Image Text:
Group 1 I H 2.2 Li 1.0 Na 0.9 K 0.8 Rb 0.8 Cs 0.8 Group 2 Be 1.6 Mg 1.3 Ca 1.0 Sr 0.9 Ba 0.9 (d-block elements) Group 13 B 2.0 Al(III) 1.6 Ga(III) 1.8 In(III) 1.8 TI(I) 1.6 TI(III) 2.0 Group 14 с 2.6 Si 1.9 Ge(IV) 2.0 Sn(II) 1.8 Sn(IV) 2.0 Pb(II) 1.9 Pb(IV) 2.3 Group 15 N 3.0 P 2.2 As(III) 2.2 Sb 2.1 Bi 2.0 Group 16 O 3.4 S 2.6 Se 2.6 Te 2.1 Po 2.0 Group 17 F 4.0 CI 3.2 Br 3.0 I 2.7 At 2.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The number of electrons in the valence shell of a central p block atom tends to control the shape of molecules Polarity is a property of molecules The net molecular dipole moment of a polyatomic speci...View the full answer

Answered By

User l_1013947
I possess a comprehensive understanding of programming languages such as C++, Python, HTML, CSS, and Jupyter Notebook. These technical skills enable me to develop robust software solutions and create visually appealing web pages. With my expertise in coding, I can effectively tackle complex programming tasks and deliver high-quality results.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
The board of directors of Portand Ltd are considering two mutually exclusive investments each of which is expected to have a life of five years. The company does not have the physical capacity to...
-
Using the data in the following table, predict the sign and magnitude of ÎH° for each of the following reactions. In each case, identify whether the reaction is expected to be endothermic...
-
Which of the following is most likely to be recognized as a defining characteristic of the public good? a) The good is a non-rival element. B) the good is a non-excludable item C) The good is both A...
-
Avery Alton practices law under the business title Avery Alton, Attorney at Law, Inc. During September, her law practice engaged in the following transactions: Sep 1 Sold $50,000 of common stock to...
-
Solve graphically. Round solutions to three decimal places, where appropriate. 10x 2 - 23x + 12 = 0
-
1 Expatriate managers need to take account of the cultural realities. Which cultural realities did the expatriate manager fail to take account of? How should he have dealt with the cultural realities?
-
Implement the following LP model in a spreadsheet. Use Solver to solve the problem and create a Sensitivity Report. Use this information to answer the following questions: MIN: 5X1 + 3X2 + 4X3...
-
Assume you are in the 42.5 percent personal tax bracket. You are considering investing $50,000 in for profit Universal Health Services (UHS) bonds that carry an 11.25 percent interest rate. a) How...
-
In the following table, match a species in list 1 with an isoelectronic partner in list 2. Some species may have more than one partner. Qualify how you have interpreted the term isoelectronic. List 1...
-
Draw Lewis structures for (a) CO 2 , (b) SO 2 , (c) OF 2 (d) H 2 CO.
-
A 12.5 mm (0.50 in.) diameter cylindrical rod fabricated from a 2014-T6 alloy (Figure 8.34) is subjected to a repeated tension-compression load cycling along its axis. Compute the maximum and minimum...
-
1. What does the phrase "cost of quality" mean? How might using this statement assist a company in addressing its quality issues? 2. What key distinctions exist between total quality human resource...
-
Does productivity in terms of output per labor our insure a company will be profitable? Why or why not? What questions should be asked to test whether productivity has increased? How do these answers...
-
How do the four Ps of marketing (product, price, promotion, place) differ in international markets?
-
Do you agree with the societal or political forces? Why or why not? Support your assertions with credible sources
-
How do the global transformational leadership models comprise a work environment that sees the need for change and embraces the new changes?Explain
-
An engineer collects the following data showing the speed and miles per gallon of a Toyota Camry. (a) Draw a scatter diagram of the data. What type of relation appears to exist between x and y? (b)...
-
Figure displays a 12.0 V battery 3 four uncharged capacitors of capacitances C1 = 4.00F, C2 = 6.00F, and C3 = 3.00F. The switch is thrown to the left side until capacitor 1 is fully charged. Then the...
-
The following Latimer diagrams show the standard reduction potentials E /V for some oxidation states of iron in acid and alkaline solution: (a) Plot a Frost diagram showing the states of Fe under...
-
Data shown below refer to redox couples for the Group 8 elements Fe and Ru. (a) Comment on the relative stability of Fe 2+ and Ru 2+ in acidic aqueous solution. (b) Give a balanced equation for the...
-
Many of the tabulated data for standard potentials have been determined from thermochemical data rather than direct electrochemical measurements of cell potentials. Carry out a calculation to...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


