In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Why is a
Question:
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed.
Why is a coupling constant measured in Hz and is not recorded as a chemical shift difference?
Table 4.3
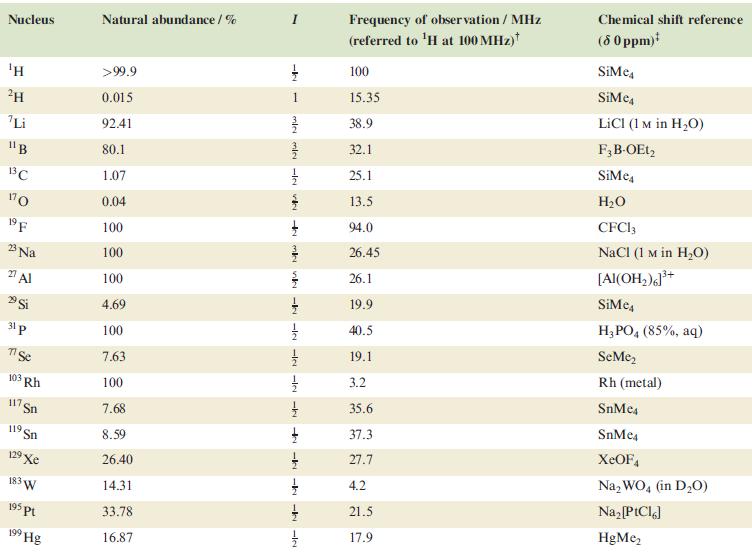
Transcribed Image Text:
Nucleus ΤΗ ²H Li 11B 13C 170 19 F 23 Na 2 Al 29 Si 31 p 7 Se 103 Rh 117. Sn 119 Sn 19 Xe 183 W 195 pt 199 Hg Natural abundance/% >99.9 0.015 92.41 80.1 1.07 0.04 100 100 100 4.69 100 7.63 100 7.68 8.59 26.40 14.31 33.78 16.87 miele v + mk nh -k -le -le-le - -ki -k -k -IN Frequency of observation / MHz (referred to ¹H at 100 MHz) 100 15.35 38.9 32.1 25.1 13.5 94.0 26.45 26.1 19.9 40.5 19.1 3.2 35.6 37.3 27.7 4.2 21.5 17.9 Chemical shift reference (8 0 ppm)* SiMe SiMe4 LICI (1 M in H₂O) F3B-OEt2 SiMe H₂O CFC13 NaCl (1 M in H₂O) [Al(OH₂)]³+ SiMc4 H3PO4 (85%, aq) SeMe₂ Rh (metal) SnMc4 SnMe4 XeOF4 Na₂WO4 (in D₂O) Na₂ [PtCl6] HgMe₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
The coupling constant in nuclear magnetic resonance NMR spectroscopy is measured in Hertz Hz because ...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. The 19 F NMR spectrum of the octahedral ion [PF5Me] shows two signals ( 45.8 and 57.6 ppm). Why are two signals...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. (a) Predict what you would expect to see in the 15 N NMR spectrum of the isotopically labelled compound cis-[Pt( 15...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Rationalize the fact that the 13 C NMR spectrum of CF 3 CO 2 H consists of two binomial quartets with coupling...
-
A common stock pays an annual dividend that increases by 4% annually and sells for $35 per share. If the market rate of return on this stock is 8%. What is the amount of the ?last dividend paid $1.53...
-
Selected account balances for Megans Mocha at the end of the month are listed below in random order: Accounts...
-
(a) Explain why the antiderivative y = e x+C is equivalent to the antiderivative y = Ce x . (b) Explain why the antiderivative y = sec x + C is equivalent to the antiderivative y = tan x + C.
-
The specification for the thickness of a piece of steel is .5 inch ; .05. The standard deviation of the band saw is .015. Using Cp, calculate whether the process is capable or not. LO.1
-
Yoto Heavy Industrial uses ten units of Part No. T305 each month in the production of large diesel engines. The cost to manufacture one unit of T305 is presented below: Direct material...
-
Substantive Testing The closing balance of inventory is $100,000 by the end of the financial year. An inventory test count by auditors revealed $30 overstatement and $15 understatement in a sample...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. How might you use 31 P NMR spectroscopy to distinguish between Ph 2 PH and Ph 3 P? Table 4.3 Nucleus H Li 11B 13C...
-
The EI mass spectrum and structure of Cr(CO) 6 is shown in Fig. 4.38. Rationalize the peaks in the spectrum. Why is the EI technique suitable for recording the mass spectrum of Cr(CO) 6 ? Figure 4.38...
-
Although we have followed convention in using the arithmetic mean of returns, for returns data, the geometric mean is often and more correctly used. The geometric mean g is defined implicitly by...
-
When in 1920 the Chia brothers opened their first shop in Bangkok selling seeds for farmers, they did not know that they were on the way to launching the development of one of the most successful...
-
In Exercises 49-52, sketch a plane. Then sketch the described situation. Three noncollinear points that lie in the plane
-
In Exercises 49-52, sketch a plane. Then sketch the described situation. A plane perpendicular to the given plane
-
Trace the polygon and point P on paper. Then draw a rotation of the polygon the given number of degrees about P. 150 F P G
-
Trace the polygon and point P on paper. Then draw a rotation of the polygon the given number of degrees about P. 30 B C
-
(a) Determine the test statistic, H, (b) Determine the critical value at the = 0.05 level of significance, (c) Test whether the distributions of the populations are different 1. 2. 13 16 12 18 14...
-
If |62x|>9, which of the following is a possible value of x? A. 2 B. 1 C. 0 D. 4 E. 7
-
In the paper Ionization energies of atoms and atomic ions (P.F. Lang and B.C. Smith, J. Chem. Educ., 2003, 80, 938) the authors discuss the apparent irregularities in the first and second ionization...
-
Calculate the wavelength of the line in the atomic spectrum of hydrogen in which n 1 = 1 and n 2 = 3. What is the energy change for this transition?
-
At various times the following two sequences have been proposed for the elements to be included in Group 3: (a) Sc, Y, La, Ac, (b) Sc, Y, Lu, Lr. Because ionic radii strongly influence the chemical...
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...

Study smarter with the SolutionInn App


