The EI mass spectrum and structure of Cr(CO) 6 is shown in Fig. 4.38. Rationalize the peaks
Question:
The EI mass spectrum and structure of Cr(CO)6 is shown in Fig. 4.38. Rationalize the peaks in the spectrum. Why is the EI technique suitable for recording the mass spectrum of Cr(CO)6?
Figure 4.38
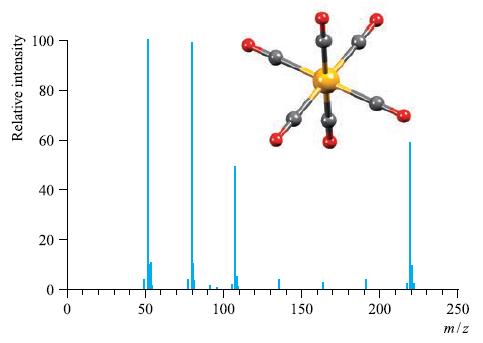
Transcribed Image Text:
Relative intensity 100 80 80 40 20 T O 50 100 150 200 T 250 m/z
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The EI mass spectrum of CrCO6 shows the following peaks A peak at mz 220 which corresponds to the mo...View the full answer

Answered By

Chandrasekhar Karri
I have tutored students in accounting at the high school and college levels. I have developed strong teaching methods, which allow me to effectively explain complex accounting concepts to students. Additionally, I am committed to helping students reach their academic goals and providing them with the necessary tools to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Rationalize the indicated fragments in the EI mass spectrum of each of the following molecules by proposing a structure of the fragment and a mechanism by which it is produced. (a)...
-
Suggest a structure for each of the ions corresponding to the following peaks in the EI mass spectrum of ethyl bromide, and give a mechanism for the formation of each ion. (The numbers in parentheses...
-
What type of pump is shown in Fig P11.5? How does it operate?
-
The annual consumption of beef per person was about 64 7 lb in 2000 and about 60 3 lb in 2007 Assume B(t), the annual beef consumption t years after 2000, is decreasing according to the exponential...
-
Suppose Pauls Hardware sells merchandise on account, terms 1/10, n/60, for $700 (cost of the inventory is $380) on March 17, 201 2. Pauls Hardware later received $235 of goods (cost, $105) as sales...
-
A 300 pound metal star is hanging on two cables which are attached to the ceiling. The left hand cable makes a 72 angle with the ceiling while the right hand cable makes a 18 angle with the ceiling....
-
The specification for the weight of a chemical in a compound is .05. If the standard deviation of the weighing scales is .02, is the process considered capable? LO.1
-
Use the following information for a manufacturer to compute cost of goods manufactured and cost of goodssold: Beginning Ending Inventories: Raw Materials Work-in-Process Finished Goods Other...
-
equirement: 1. what is the relationship between the classes in the below UML diagram? 2. List the tables with primary keys, attributes, and foreign keys that implement the following UML Class diagram...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. Why is a coupling constant measured in Hz and is not recorded as a chemical shift difference? Table 4.3 Nucleus H...
-
Both positive and negative-ion ESI mass spectra of [Me 4 Sb][Ph 2 SbCl 4 ] were recorded. In one spectrum, peaks at m/z 181 (100%), 182 (4.5%), 183 (74.6%) and 184 (3.4%) were observed. The other...
-
Gilles Corp. purchased a piece of equipment on February 1, 2023, for $100,000. The equipment has an estimated useful life of eight years, with a residual value of $25,000, and an estimated physical...
-
The equation for the standard normal curve (the normal curve with mean 0 and standard deviation 1) graphs as an exponential curve. Graph this curve, whose equation is \[y=\frac{e^{-x^{2} /...
-
Design an undirected network with N=7 and L=12. Based on how you drew your network, classify it as either fully connected ,random, or scale-free. Justify your decision with a short paragraph response.
-
Use the Ch08_AviaCo database shown in Figure P8.35 to work Problems 3546. Modify the MODEL table to add the attribute and insert the values shown in the following table. Table P8.35 Attribute and...
-
The Tip Calculator app does not need a Button to perform its calculations. Reimplement this app to use property listeners to perform the calculations whenever the user modifies the bill amount or...
-
A particle, carrying a positive charge of \(4 \mathrm{nC}\), located at \((5 \mathrm{~cm}, 0)\) on the \(x\)-axis experiences an attractive force of magnitude 115.2 \(\mathrm{N}\) due to an unknown...
-
Every year Money magazine publishes its list of top places to live. The following data represent a list of top places to live for a recent year, along with the median family income and median commute...
-
Discuss the information available from the following techniques in the analysis of inorganic pigments used in antique oil paintings: (i) Powder X-ray diffraction, (ii) Infrared and Raman...
-
When 58.4 nm radiation from a helium discharge lamp is directed on a sample of krypton, electrons are ejected with a velocity of 1.59 10 6 m s 1 . The same radiation ejects electrons from Rb atoms...
-
During 1999 several papers appeared in the scientific literature claiming that d orbitals of Cu 2 O had been observed experimentally. In his paper Have orbitals really been observed? (J. Chem. Educ.,...
-
Show that the following four lines in the Lyman series can be predicted from eqn 1.1: 91.127, 97.202, 102.52, and 121.57 nm. Equation 1.1. 2 R 1 n n (1.1)
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


