The electronic absorption spectrum of [Co(OH 2 ) 6 ] 2+ exhibits bands at 8100, 16000 and
Question:
The electronic absorption spectrum of [Co(OH2)6]2+ exhibits bands at 8100, 16000 and 19400 cm–1.
(a) Assign these bands to electronic transitions.
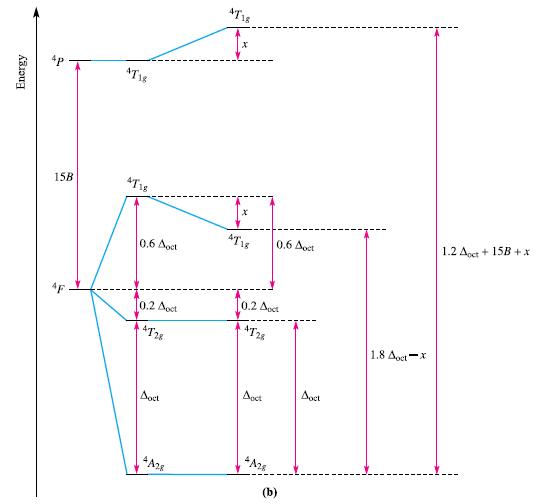
(b) The value of Δoct for [Co(OH2)6]2+ listed in Table 20.2 is 9300 cm–1. What value of Δoct would you obtain using the diagram in Fig. 20.23b? Why does the calculated value not match that in Table 20.2?
Table 20.2.
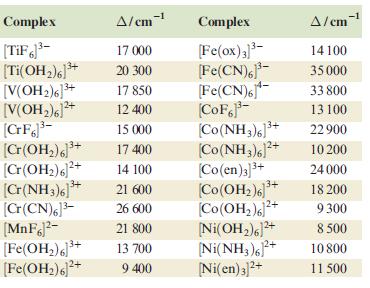
Figure 20.23(b).
Transcribed Image Text:
Complex [TiF6³- [Ti(OH₂)6]³+ [V(OH₂)6]³+ [V(OH₂)]²+ [CrF6³- 13+ [Cr(OH₂)6]³+ [Cr(OH₂)6]²+ [Cr(NH3)6]³+ [Cr (CN)6]³- [MnF²- [Fe(OH₂)6³ [Fe(OH₂)6]²+ 12+ 13+ A/cm-¹ 17 000 20 300 17 850 12 400 15 000 17 400 14 100 21 600 26 600 21 800 13 700 9 400 Complex [Fe(ox) 3³- 13- [Fe(CN)6]³- [Fe(CN)6]¹ [CoF6³- [Co(NH3)6]³+ [Co(NH3)6]2+ [Co(en)3]³+ 13+ 12+ [Co(OH₂)6] [Co(OH₂)6] [Ni(OH₂)6]²+ [Ni(NH3)62 [Ni(en)3]²+ 12+ A/cm-¹ 14100 35.000 33 800 13 100 22900 10 200 24000 18 200 9300 8 500 10 800 11 500
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a V 1 3 T1g 3 T 2g 8100 cm 1 V 2 3 T1g 3A 2g 16000 cm 1 ...View the full answer

Answered By

User l_1013947
I possess a comprehensive understanding of programming languages such as C++, Python, HTML, CSS, and Jupyter Notebook. These technical skills enable me to develop robust software solutions and create visually appealing web pages. With my expertise in coding, I can effectively tackle complex programming tasks and deliver high-quality results.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Problems 2738, the reduced row echelon form of a system of linear equations is given. Write the system of equations corresponding to the given matrix. Use x, y; or x, y, z; or x 1 , x 2 , x 3 , x...
-
The emission spectrum of a porphyry in dissolved in O,-saturated water shows a strong band at 650 nm and a weak band at 1270 nm. In separate experiments, it was observed that the electronic...
-
(a) The values of max for the most intense absorptions in the electronic spectra of [CoCl 4 ] 2 and [Co(OH 2 ) 6 ] 2+ differ by a factor of about 100. Comment on this observation and state which...
-
The equity profolio of a company is thus 31/12/2001. Nature: Actions A, Quantity = 350, Unit cost = 36000, Purchase dates = 03/1999, 2000 = 37000, 2001= 34000. Nature: Actions B, Qauntity = 125, Unit...
-
Do the concepts of complexity and divergence apply to an online sales company such as Dell Computer?
-
Show, by reference to a figure, that the angle between the tangents to two curves at a point of intersection may be found from the formula When will the two curves intersect at right angles? tan...
-
Microsoft has undoubtedly been the most successful software firm ever. Between 1994 and 2000, the firm's revenues increased from $2.8 billion to $23.0 billion, and its earnings from $708 million to...
-
The general ledger of Stephens Products, Inc. contains the following control account: If the materials charged to the one uncompleted job still in process amounted to $3,400, what amount of labor and...
-
Jansen Company's adjusted trial balance as of December 31, 2020 is below. What is the balance in retained earnings after the books are closed on December 31.20202 Debits Credits Cash Prepaid rent...
-
For which member of the following pairs of complexes would oct be the larger and why: (a) [Cr(OH 2 ) 6 ] 2+ and [Cr(OH 2 ) 6 ] 3+ ; (b) [CrF 6 ] 3 and [Cr(NH 3 ) 6 ] 3+ ; (c) [Fe(CN) 6 ] 4 and...
-
Arrange the following ligands in order of increasing field strength: Br , F , [CN] , NH 3 , [OH] , H 2 O.
-
From the Data Bank, choose 10 values from any variable, construct a boxplot, and interpret the results. Data from Data Bank Data Bank 088989 ID number 01 02 03 04 05 06 07 Age 27 18 32 24 19 56 65 36...
-
Piperel Lake Resort's four employees are paid monthly. Assume an income tax rate of 20%. Required: Complete the payroll register below for the month ended January 31, 2021. (Do not round intermediate...
-
4. [Communication network 1] Consider the communication network shown in the figure below and suppose that each link can fail with probability p. Assume that failures of different links are...
-
The 60 deg strain gauge rosette is mounted on the bracket. The following readings are obtained for each gauge a = 100 106, b = 250 106, and c = 150 106. Determine: (a) the strains x, y, and xy for...
-
Assume the ledge has the dimensions shown and is attached to the building with a series of equally spaved pins around the circumference of the building. Design the pins so that the ledge can support...
-
Describe in detail about the arterial supply and venous drainage of heart with its Applied Anatomy?
-
The consolidated 2019 and 2018 balance sheets for Butler Corporation follow. Butler Corporation Consolidated Balance Sheets (In millions except per share amounts) Required: 1. Calculate the...
-
As you rewrite these sentences, replace the cliches and buzzwords with plain language (if you don't recognize any of these terms, you can find definitions online): a. Being a jack-of-all-trades, Dave...
-
The regions of + in a compound are the regions most likely to be attacked by an anion, such as hydroxide (HO - ). In the compound below, identify the two carbon atoms that are most likely to be...
-
Consider the three compounds shown below and then answer the questions that follow: a) Which two compounds are constitutional isomers? b) Which compound contains a nitrogen atom with trigonal...
-
Compound A is an alkene that was treated with ozone (followed by DMS) to yield only 4-heptanone. Identify the major product that is expected when compound A is treated with MCPBA followed by aqueous...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


