Prove equation (3.19). Also prove that n! = ?(2 n )?and?n!?=?o(n n ). Equation (3.19) (2) Ig(n!)
Question:
Prove equation (3.19). Also prove that n! = ?(2n)?and?n!?=?o(nn).
Equation (3.19)
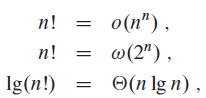 (2") Ig(n!) O(n lg n) , || || ||" style="" class="fr-fic fr-dib">
(2") Ig(n!) O(n lg n) , || || ||" style="" class="fr-fic fr-dib">
Transcribed Image Text:
п! о (п"), n! >(2") Ig(n!) O(n lg n) , || || ||
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
1 We have n n n1 n2 2 1 n n n n n n n n So n on n 2Similarly n n n1 n2 2 1 2 2 2 ...View the full answer

Answered By

Suvojit Dhara
I am a Ph.D student in Mathematics. I have taught maths as a private tutor for class - XI,XII students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction to Algorithms
ISBN: 978-0262033848
3rd edition
Authors: Thomas H. Cormen, Charles E. Leiserson, Ronald L. Rivest
Question Posted:
Students also viewed these Computer science questions
-
We have seen how to evaluate a polynomial of degree-bound n at a single point in O(n) time using Horner's rule. We have also discovered how to evaluate such a polynomial at all n complex roots of...
-
Prove each of the following statements. a) 2n + 1 < 2n for n = 3, 4,.... b) n < 2n for n = 1, 2,.... c) n2 < 2n + 1 for n = 1, 2,.... d) n3 < 3n for n = 1, 2,....
-
Prove that for all n Z+, n > 3 2n < n.
-
True or False Financial ratios are the principal tools of financial analysis because they standardize financial information so that comparisons can be made between firms of varying sizes.
-
Using the information in Figure 16-2, calculate the values of Ho for the following reactions: In Figure 16.2 (a) (b) (c) (-359 predicted) (-240 predicted) resonance enerty encrgy energy 240 kJ/mol232...
-
Select one major public or private payer for healthcare services and list all the conditions that the primary care provider must meet to qualify for reimbursement.
-
43. When a taxpayer receives a nonqualified distribution from a Roth IRA, is the entire amount of the distribution treated as taxable income?
-
The following data were drawn from the CAFRs of two northern Virginia cities (all dollar amounts are in thousands): a. Per capita total general-fund taxes? b. Per capita property taxes? c. Tax rate...
-
Munoz Sporting Equipment manufactures baseball bats and tennis rackets. Department B produces the baseball bats, and Department T produces the tennis rackets. Munoz currently uses plantwide...
-
The proposed rates were not in the range the CEO expected given the pricing analysis. The CEO has asked the pricing actuary to verify the total projected loss cost excluding potential large storm...
-
Indicate, for each pair of expressions (A, B) in the table below, whether A is O, o, ? , ?, or ? of B. Assume that k ? 1, ? > 0, and c > 1 are constants. Your answer should be in the form of the...
-
Let f (n) an= g(n) be asymptotically positive functions. Prove or disprove each of the following conjectures. a. f (n) = O(g(n)) implies g(n) = O(f (n)). b. f (n) + g(n) = (min(f (n), g(n))). c. f...
-
Strontium-90 has a half-life of 28 days. (a) A sample has initial mass 50 mg. Find a formula for the mass remaining after t days. (b) Find the mass remaining after 40 days. (c) How long does it take...
-
1 . Journalize the following transactions: ( a ) Issued 1 , 0 0 0 shares of $ 1 0 par common stock at $ 5 9 for cash. ( b ) Issued 1 , 4 0 0 shares of $ 1 0 par common stock in exchange for equipment...
-
Using alpha .05, determine if moving to a larger enclosure decreased tiger anxiety levels. You should first calculate the difference (After - Before) Tiger Before Anthony 45 45 Banthony 56 After 38...
-
Cyclohexane (C 6 H 12 ) is produced by mixing Benzene and hydrogen. A process including a reactor, separator, and recycle stream is used to produce Cyclohexane. The fresh feed contains 260L/min C 6 H...
-
Suppose the city is undergoing severe ination. Specifically, both goods prices have risen by 10%. What percentage of a raise in the wage rate should Alex request from her boss, for her to maintain...
-
1. An iron cube of mass 0.55 kg is raised to a temperature of 100C by being placed in boiling water for 5 minutes. It is then removed and transferred immediately to an aluminium calorimeter filled...
-
Among the following choices, the group that activates the benzene ring toward electrophilic aromatic substitution is (a) NO 2 ; (b) 2CF 3 ; (c) CO 2 H; (d) OCH 3 ; (e) Br.
-
True & False The basis of an asset must be reduced by the depreciation allowable, 2. Adjusted gross income (AGI) is the basis for a number of phase-outs of deductions. 3. A change to adjusted gross...
-
Give an implementation of the stack ADT using an array list for storage.
-
Consider an implementation of the array list ADT using a dynamic array, but instead of copying the elements into an array of double the size (that is, fromN to 2N) when its capacity is reached, we...
-
Describe how to implement a method, alternateIterator( ), for a positional list that returns an iterator that reports only those elements having even index in the list.
-
Which investment should I choose? Bond A: BBB Corporate bond, Price=$1,100, Par=$1,000, Coupon rate=4% (semiannual coupons), 13 years to maturity Bond B: BBB Corporate bond, Price=$5,900, Par=$5,000,...
-
During October, total equivalent units of output were 86,000 using the weighted-average method. The information about the beginning and ending inventories for October were as follows: Units in...
-
Q12. PDQ has an expected sales volume of $1,000,000 with a variable cost ratio of 55%, and fixed costs of $200,000. What sales volume would be necessary to achieve a $100,000 after-tax profit when...

Study smarter with the SolutionInn App


