(a) The molar Gibbs energy of mixing (per mole of superficial solution) for a liquid binary system...
Question:
(a) The molar Gibbs energy of mixing (per mole of superficial solution) for a liquid binary system![]()
expressed extensively, this becomes![]()
Introduce the concepts of chemical theory into Eqn. 19.131 to prove that the Gibbs energy of mixing is equivalently given by the sums over true species,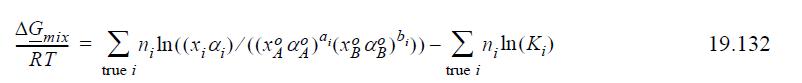
where Ki is unity for the monomers.
(b) Show that on a molar basis for an ideal chemical theory solution that has only solvation, per mole of true solution, the equation reduces to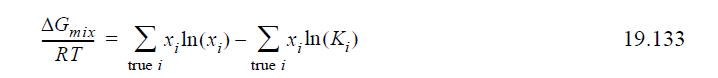
and provide a physical interpretation relating the Gibbs energy of formation to K.
(c) Considering a system where A associates, show that the Gibbs energy of mixing by ideal chemical theory is per mole of true solution given by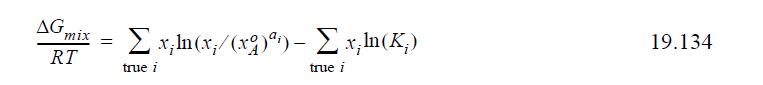
Below are tabulated calculations for ideal chemical theory for an A + B system where A forms dimers with K=140. Use Eqns. 19.134 and 19.130 to tabulate the respective Gibbs energies of mixing over RT. Then tabulate nT0 (the number of true moles divided by the number of apparent moles) and multiply Eqn. 19.134 by this number and compare with Eqn. 19.130.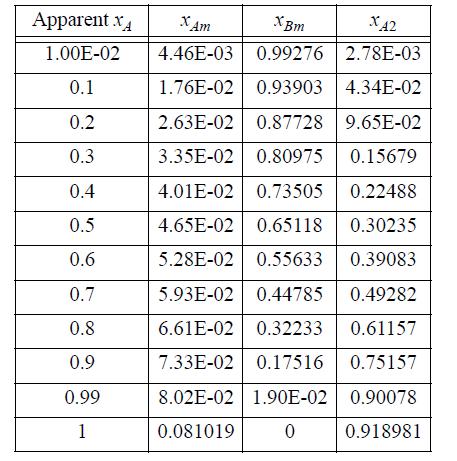
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





