Air, even more than carbon dioxide, is inexpensive and nontoxic. Why is it not the gas of
Question:
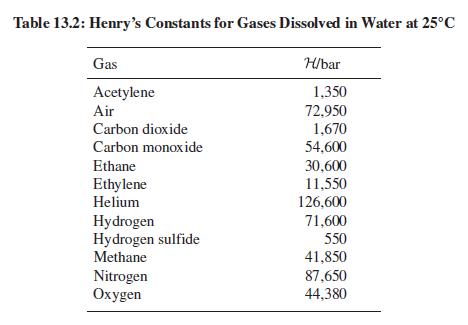
Air, even more than carbon dioxide, is inexpensive and nontoxic. Why is it not the gas of choice for making soda water and (cheap) champagne effervescent? Table 13.2 may provide useful data.
Table 13.2
Transcribed Image Text:
Table 13.2: Henry's Constants for Gases Dissolved in Water at 25°C Gas H/bar Acetylene 1,350 72,950 1,670 Air Carbon dioxide Carbon monoxide 54,600 Ethane 30,600 Ethylene Helium 11,550 126,600 Hydrogen Hydrogen sulfide Methane 71,600 550 Nitrogen Охудen 41,850 87,650 44,380
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
ANSWER The effervescence in soda water and champagne is caused by the presence of dissolved carbon dioxide gas While air is indeed inexpensive and non...View the full answer

Answered By

Chegwek Alex
As a tutor, I have gained extensive hands-on experience and proficiency in providing personalized and effective academic support to students of all ages and skill levels. I have worked with students in various subjects, including but not limited to mathematics, science, language arts, and social studies.
I have a strong educational background, having obtained a degree in [your field of study] from [university name]. I have also undergone rigorous training and professional development programs to improve my teaching skills and stay up-to-date with the latest trends and developments in education.
I am adept at creating individualized learning plans that cater to each student's unique learning style, strengths, and weaknesses. I use a variety of teaching strategies, including visual aids, interactive exercises, and hands-on activities, to engage students and help them achieve their academic goals.
In addition to subject-specific instruction, I also provide guidance on study skills, time management, and test-taking strategies, equipping students with the tools they need to become successful, independent learners.
I have a proven track record of success, with many of my students achieving significant improvements in their grades and academic performance. I am a dedicated and passionate tutor, committed to providing personalized, effective, and engaging tutoring services to help students achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
Carbon dioxide is stripped from water by air in a wetted-wall tube. At a certain location, where the pressure is 10 atM and the temperature is 25oC, the mass-transfer flux of CO2 is 1.62 Ibmouh-ft2....
-
Carbon dioxide gas at 1 atmosphere pressure it to be heated from 25?C to 75?C by pumping it through a tube bank at a velocity of 4 m/s. The tubes are heated by steam condensing within them at 200?C....
-
Carbon dioxide gas enters a pipe at 3 MPa and 500 K at a rate of 2 kg/s. CO2 is cooled at constant pressure as it flows in the pipe and the temperature of CO2 drops to 450 K at the exit. Determine...
-
Given the following information set up the problem in a transportation table and solve for the minimum-cost plan: Minimum total cost? Demand 550 700 750 Capacity 500 500 500 Regular Overtime 50 50...
-
What does it mean to say that a person has a low positive rate of time preference as opposed to having a high positive rate of time preference?
-
Enormo Corporation is a large multinational audit client of your CPA firm. One of Enormos subsidiaries, Ultro, Ltd., is a successful electronics assembly company that operates in a small Caribbean...
-
Horatio and Kelly were divorced at the end of last year. Neither Horatio nor Kelly remarried during the current year and Horatio moved out of state. Determine the filing status of Horatio and Kelly...
-
Omega, Inc., a publicly held corporation, has assets of $100 million and annual earnings in the range of $13 to $15 million. Omega owns three aluminum plants, which are profitable, and one plastics...
-
I want a quick solution without explanation Question 25 Not yet answered Marked out of 1.00 Flag question Which one of the following statements said by the prospect represents the prospects...
-
Economist Daron Acemoglu of the Massachusetts Institute of Technology has written extensively about the role of political institutions and economic growth. Acemoglu distinguishes broadly between two...
-
Consider an ethanol(1)/ethyl acetate(2) mixture with x 1 = 0.20, initially at 70C and 100 kPa. Describe the evolution of phases and phase compositions as the temperature is gradually increased to...
-
A mixture of ethanol and ethyl acetate is heated in a closed system at 100 kPa to a temperature of 74C, and two phases are observed to be present. What are the possible compositions of the liquid and...
-
Lisa Montgomery and Joel Chalmers established a coffee bean distribution business. Their partnership shared profits and losses based on an agreement that gave Lisa a salary allowance of $45,000 and...
-
IFRS Financial Statements Thomson Reuters is a global information company created by the 2008 merger of the Thomson Corporation, a Canadian company, with the Reuters Company, a United Kingdom-based...
-
Burgess Services Co. experienced the following events in 2011: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
In Exercises 13 and 14, use the box-and-whisker plot to identify the five-number summary. 0 2 5 8 10 ++ ++ 0 1 2 3 4 5 6 7 8 9 10 11
-
In a test of the effect of dampness on electrical connections, 80 electrical connections were tested under damp conditions and 130 were tested under dry conditions. Twenty of the damp connections...
-
Zelta Ltd. is a medium-size company involved in providing a range of specialized products and services for the aerospace industry. Just over a year ago, external consultants undertook a major review...
-
The following account balances relate to the stockholders equity accounts of Molder Corp. at year-end. A small stock dividend was declared and issued in 2022. The market price of the shares was...
-
Big Jim Company sponsored a picnic for employees and purchased a propane grill equipped with a standard-sized propane tank for the picnic. To make sure there was enough propane for all the cooking...
-
Benzyl chloride is manufactured by the thermal or photochemical chlorination of toluene. The chlorination is usually carried out to no more than 50% toluene conversion to minimize the benzyl chloride...
-
A pharmaceutical product is crystallized and washed with absolute ethanol. A 100 kg batch of product containing 10% ethanol by weight is to be dried to 0.1% ethanol by weight by passing 0.2 m 3 /min...
-
Solvent vessels must be purged before maintenance personnel enter in order to ensure that: (1) Sufficient oxygen is available for breathing; (2) Vapor concentrations are below the flash point; (3)...
-
Kenneth lived in his home for the entire year except for when he rented his home (near a very nice ski resort) to a married couple for 14 days in December. The couple paid Kenneth $14,000 in rent for...
-
On December 31, 2021, Shack Store Inc had 143 million shares outstanding, which traded for $643.29 per share. On January 02, 2022, the CEO announced a 20-for-1 stock split. Every shareholder would...
-
o1s= secom o1s= secom

Study smarter with the SolutionInn App


