A mixture of ethanol and ethyl acetate is heated in a closed system at 100 kPa to
Question:
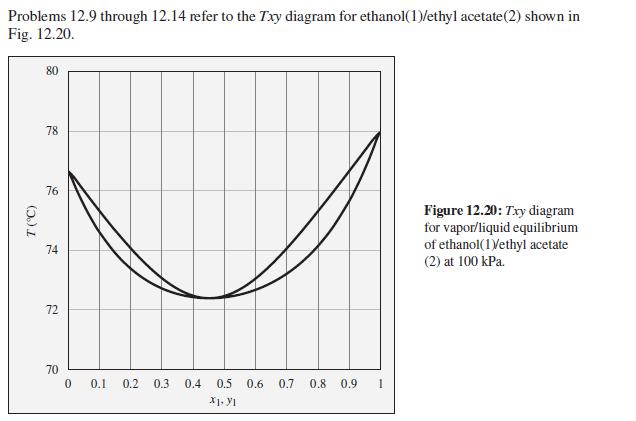
A mixture of ethanol and ethyl acetate is heated in a closed system at 100 kPa to a temperature of 74°C, and two phases are observed to be present. What are the possible compositions of the liquid and vapor phases?
Transcribed Image Text:
Problems 12.9 through 12.14 refer to the Txy diagram for ethanol(1)/ethyl acetate(2) shown in Fig. 12.20. 80 78 76 Figure 12.20: Txy diagram for vapor/liquid equilibrium of ethanol(1Vethyl acetate 74 (2) at 100 kPa. 72 70 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 X1. YI (5.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
The pressure above a mixture of ethanol and ethyl acetate at 70C is measured to be 78 kPa. What are the possible compositions of the liquid and vapor phases? Problems 12.3 through 12.8 refer to the...
-
The pressure above a mixture of ethanol and ethyl acetate at 70C is measured to be 86 kPa. What are the possible compositions of the liquid and vapor phases? Problems 12.3 through 12.8 refer to the...
-
A chloroform and tetrahydrofuran mixture is heated in a closed system at 120 kPa to a temperature of 70C, and two phases are observed to be present. What are the possible compositions of the liquid...
-
Solve Utt = cUTT 0
-
Denise will receive $1,000 in two years. If the interest rate is 5 percent, what is the present value of this dollar amount?
-
Three months ago, a national public accounting firm hired Greg Scott to work as staff auditor in its New York office. Yesterday, Scott's father was hired to be the chief financial officer of one of...
-
The couple has been living apart for the last two years and both children live with Kano. Kano has provided all the means necessary to support himself and his children. Kano and Hoshi do not file a...
-
The nancial statements of the Lance Armstrong Foundation (see Table 128) contain the following note titled Promises to Give: Unconditional promises to give were as follows at December 31: The...
-
Explain master budget and the advantages of implementing budgets . ( 1 5 marks )
-
A ride hailing company has their DB structured in 3 major tables as described in the SCHEMA section below. Write a query to fetch the top 100 users who traveled the most distance using the service....
-
Air, even more than carbon dioxide, is inexpensive and nontoxic. Why is it not the gas of choice for making soda water and (cheap) champagne effervescent? Table 13.2 may provide useful data. Table...
-
A mixture of ethanol and ethyl acetate is heated in a closed system at 100 kPa to a temperature of 77C, and two phases are observed to be present. What are the possible compositions of the liquid and...
-
Would AT&T and Microsoft use the cash basis or the accrual basis of accounting? Explain.
-
Using a ruler and set squares only, construct the following shapes: a. b. c. d. 5cm 5cm
-
The marketing department has just forecast that 10,000 units of item 778 will be ordered in the next fiscal year. Based on the marketing department's forecast and noting that the seasonal relative...
-
Following are interaction plots for three data sets. Which data set has the largest interactions? Which has the smallest? A B C
-
From your local chamber of commerce, obtain the population figures for your city for the years \(1980,1990,2000\), and 2010. Find the rate of growth for each period. Forecast the population of your...
-
A mass \(m\) is attached at the midpoint of a stretched wire of area of cross-section \(A\), length \(l\), and Young's modulus \(E\) as shown in Fig. 13.29. If the initial tension in the wire is...
-
You are provided with the following information regarding events that occurred at Moore Corporation during 2022 or changes in account balances as of December 31, 2022. Instructions Moore prepares its...
-
A genetically engineered strain of Escherichia coli (E. coli) is used to synthesize human insulin for people suffering from type I diabetes mellitus. In the following simplified reaction scheme,...
-
An easy way to rationalize definitions of cycle performance is to think of them as: Thus, for an engine, thermal efficiency is =|W|/|Q H |; for a refrigerator, the coefficient of performance is =|Q C...
-
Devise a general scheme for analyzing four-step air-standard power cycles. Model each step of the cycle as a polytropic process described by PV = constant which implies that TP (1 ) = constant...
-
Air-standard power cycles are conventionally displayed on PV diagrams. An alternative is the PT diagram. Sketch air-standard cycles on PT diagrams for the following: (a) Carnot cycle (b) Otto cycle...
-
On April 1, year 1, Mary borrowed $200,000 to refinance the original mortgage on her principal residence. Mary paid 3 points to reduce her interest rate from 6 percent to 5 percent. The loan is for a...
-
Give a numerical example of: A) Current liabilities. B) Long-term liabilities?
-
Question Wonder Works Pte Ltd ( ' WW ' ) produces ceramic hair curlers to sell to department stores. The production equipment costs WW $ 7 0 , 0 0 0 four years ago. Currently, the net book value...

Study smarter with the SolutionInn App


