Consider a Markov chain with two possible states, S = {0, 1}. In particular, suppose that the
Question:
Consider a Markov chain with two possible states, S = {0, 1}. In particular, suppose that the transition matrix is given by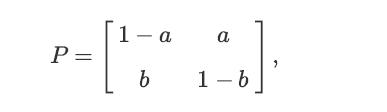
where a and b are two real numbers in the interval [0, 1] such that 0
where α ∈ [0, 1].
a. Using induction (or any other method), show that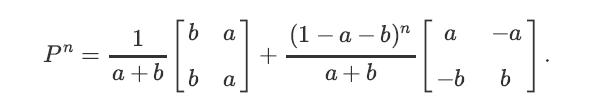
b. Show that![lim Pn = pn n 1 b +[a] a+b b](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/3/8/8/574653b5a5e86cdd1698388571794.jpg)
c. Show that![lim 7(n) n [+] = a+b a+b](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1698/3/8/8/586653b5a6a89b351698388584654.jpg)
Transcribed Image Text:
P = 1-a a b 1-b
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a For n 1 we have p pn1 PP a b b 1 ab b a 1b a a a 1ab ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introduction To Probability Statistics And Random Processes
ISBN: 9780990637202
1st Edition
Authors: Hossein Pishro-Nik
Question Posted:
Students also viewed these Business questions
-
Consider a Markov chain with two possible states s1 and s2 and with stationary transition probabilities as given in the following transition matrix P: where the value of is unknown (0 1). Suppose...
-
Consider a Markov chain in Example 11.12: a Markov chain with two possible states, S = {0, 1}, and the transition matrix where a and b are two real numbers in the interval [0, 1] such that 0 0 and r...
-
Consider a Markov chain in Example 11.12: a Markov chain with two possible states, S = {0, 1}, and the transition matrix where a and b are two real numbers in the interval [0, 1] such that 0 Example...
-
Physical Science the table shows equivalent Fahrenheit and Celsius temperatures. Degrees Fahrenheit Degrees Celsius 32 ................... 0 68 ................. 20 104 ................. 40 140...
-
Gigi LeBlanc is an advertising consultant who tracks costs for her jobs using a job order costing system. During September, LeBlanc and her staff worked on and completed jobs for the following...
-
Suad Alwan, the purchasing agent for Dubai Airlines, is interested in determining what he can expect to pay for airplane number 4 if the third plane took 20,000 hours to produce. What would Alwan...
-
What is the exemplar approach to categorization? How does it differ from the prototype approach, and how might the two approaches work together? L01
-
An employee earns $60 per hour and 1.5 times that rate for all hours in excess of 40 hours per week. Assume that the employee worked 55 hours during the week, Assume further that the social security...
-
The state government established a capital project fund in 2019 to build new highways. The fund is supported by a 5 percent tax on diesel fuel sales in the state. The tax is collected by private gas...
-
Consider the Markov chain shown in Figure 11.13. Let t k be the expected number of steps until the chain hits state 1 for the first time, given that X 0 = k. Clearly, t 1 = 0. Also, let r 1 be the...
-
Consider the Markov chain in Figure 11.12. Let's define b i as the absorption probability in state 3 if we start from state i. Use the above procedure to obtain b i for i = 0, 1, 2, 3. 13 1 co/N 3 2...
-
Lisa Meilo works for Pacific Company, which pays its employees time- and- a- half for all hours worked in excess of 40 per week. Meilos pay rate is $ 37 per hour. Her wages are subject to federal...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
9. For diatomic molecules, the correct statement(s) about the molecular orbitals formed by the overlap of two 2pz orbitals is (are) (A) orbital has a total of two nodal planes. (B) * orbital has one...
-
12. The treatment of galena with HNO3 produces a gas that is (A) paramagnetic (C) an acidic oxide (B) bent in geometry (D) colorless
-
The Mesa Redbirds football team plays in a stadium with a seating capacity of 80,000. However, during the past season, attendance averaged only 50,000. The average ticket price was $30. If price...
-
1. As a general strategy, would you recommend that Carl take an aggressive approach to capacity expansion or more of a wait-and-see approach? 2. Should Carl go with the option for one facility that...
-
Mr. and Mrs. Pickens purchased a used piano in Y1 for their young son who had started taking piano lessons. In Y8 while cleaning the piano, Mrs. Pickens discovered $4,800 of old currency. They...
-
Lisa and John are in the business of breeding beavers to produce fur for sale. They recently purchased a pair of breeding beavers for $30,000 from XUN, Inc., and agreed to pay interest at 10% each...
-
Mike, a real estate broker in California, recently inherited a farm from his deceased uncle and plans to sell the farm to the first available buyer. His uncle purchased the property 12 years ago for...
-
what is common stock and retained earnings? what i have is wrong mathxt.com Acct 2300 LI-Flnanclal Accounting Spring 2020 Homework: HW Ch 1 HW Score 6 of 7 (6 complete) Score: 0.96 of1 pt E1-31A...
-
The company Omega has the following information at the end of the reporting period: Payments to acquire new fixed assets in the amount of 239 700 Depreciation expense for the reporting period...
-
Kenworth Company uses a job-order costing system. Only three jobs-Job 105, Job 106, and Job 107-were worked on during November and December. Job 105 was completed on December 10; the other two jobs...

Study smarter with the SolutionInn App


