Using your knowledge of intermolecular forces, explain the following observation: (a) At 300C and 30 bar, the
Question:
Using your knowledge of intermolecular forces, explain the following observation:
(a) At 300°C and 30 bar, the internal energy of water is less than at 300°C and 20 bar.
(b) At 300 K and 30 bar, the compressibility factor of isopropanol (H3CCOHCH3) is less than that of n-pentane (C5H12), but at 500 K and 30 bar, the compressibility factor of isopropanol (H3CCOHCH3) is greater than that of n-pentane (C5H12).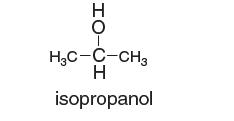
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





