(a) How much energy would be necessary to take a hydrogen atom from its ground state to...
Question:
(a) How much energy would be necessary to take a hydrogen atom from its ground state to an excited state in which the n = 3 orbit is occupied?
(b) What would be the energy of light emitted when the excited atom relaxed back to the ground state?
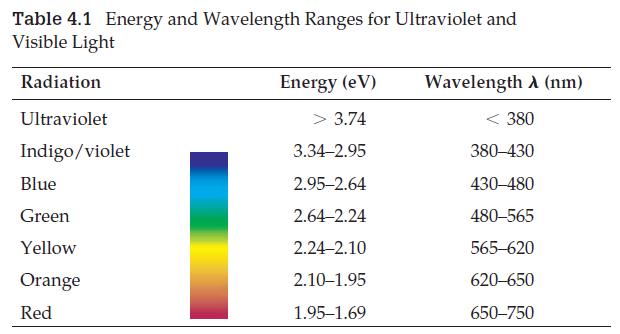
(c) What kind of electromagnetic radiation would be emitted? (Consult Table 4.1.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





