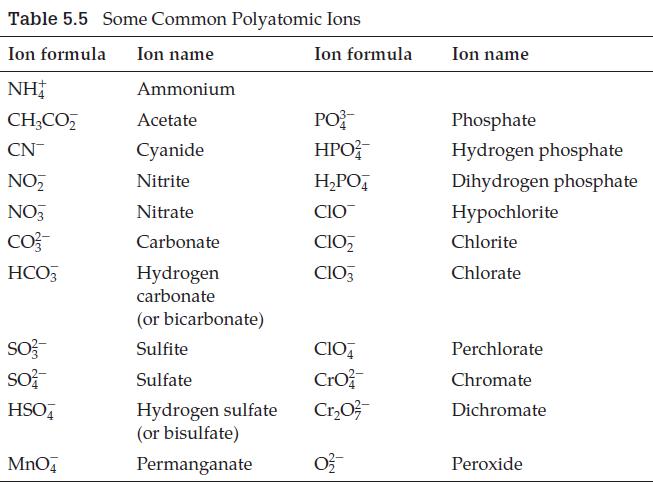
A polyatomic ion not listed in Table 5.5 is iodate, IO 3 . What is the
Question:
A polyatomic ion not listed in Table 5.5 is iodate, IO3–. What is the formula for the periodate ion, and what is the formula for magnesium periodate?
Transcribed Image Text:
Table 5.5 Some Common Polyatomic Ions Ion formula Ion name NH Ammonium CH3CO₂ Acetate CN Cyanide NO₂ Nitrite NO3 Nitrate CO²- Carbonate HCO3 Hydrogen carbonate SO3- SO² HSO4 MnO4 (or bicarbonate) Sulfite Sulfate Hydrogen sulfate (or bisulfate) Permanganate Ion formula PO HPO²/ H₂PO4 CIO CIO₂ CIO3 CIO4 Cro Cr₂0²/ 0²2/ Ion name Phosphate Hydrogen phosphate Dihydrogen phosphate Hypochlorite Chlorite Chlorate Perchlorate Chromate Dichromate Peroxide
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The formula for the periodate ion is IO4 The periodate i...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Examine the processes in Example 13.2 One of the processes that was rejected in the second round of decision making has great potential for making the integral bladed hub for the fan from an aluminum...
-
The iodate ion is reduced by sulfite according to the following reaction: The rate of this reaction is found to be first order in IO3-, first order in SO32-, and first order in H+. (a) Write the rate...
-
Let A = [a;l E Fxn be a square n x n matrix. We define the trace of A to be the number Tr(A) = akk- k=1 a) Prove that Tr : FXn F is a linear function. b) Let A, B e F"X". Prove that Tr(AB) = Tr(BA)....
-
DoorDash acquired 51% of the common stock of Wolt on December 31, 2021, for $8,100,000,000. At the date of acquisition, Wolt reported common stock with a par value of $10,000,000,000, additional...
-
In tabular form, compare the total materials cost transferred to Work in Process and the cost of the ending inventory for each method used in E2-6, E2-7, and E2-8. Discuss the effect that each method...
-
A summary of data from the income statements and balance sheets for Okumura Construction Supply Company for 20x8 and 20x7 appears below. Total assets and owner's equity at the beginning of 20x7 were...
-
Medford Company has seven operating segments but only four (G, H, I, and J) are of significant size to warrant separate disclosure. As a whole, the segments generated revenues of $710,000 ($520,000 +...
-
Determine the annual financing cost of a 1-year (365 day), $10,000 discounted bank loan at a stated annual interest rate of 9.5 percent. Assume that no compensating balance is required.
-
ALL I NEED HELP WITH IS COMPLETING THE PROBLEM I GOT WRONG. IT IS WRONG AND INCOMPLETED D Required information [The following information applies to the questions displayed below.] At the beginning...
-
The oxide ion is O 2 . How does this ion differ from the peroxide ion, O 2 2 ? Draw dot diagrams for both.
-
Consider the sulfate, sulfite, nitrate, nitrite, chlorate, and chlorite ions. What information do the -ate and -ite suffixes communicate?
-
Explain the asymmetric information theory of capital structure.
-
Q3. (a) Sketch and name the road structure layers including the bituminous coating layers. State the material's CBR value and the degree of compaction (DOC) for each road layer required by road works...
-
2. Justify the following in terms of impulse and momentum: a. Why are padded dashboards safer in automobiles?
-
The kidneys are an essential organ to regulating blood pressure in the cardiovascular system. Renal epithelial cells feature cilia on their surface, which allows them to sense blood flow in the...
-
Novak Corp. supplies its customers with high-quality canvas tents. These canvas tents sell for $170 each, with the following DM and DL usage and price expectations. Direct materials 9 square yards...
-
Develop a one-week schedule for your Housekeeping employees. Using the Excel spreadsheet that is attached above, you will prepare a schedule for the week The day-by-day occupied rooms forecast has...
-
The wave equation of physics is the partial differential equation where c is a constant. Show that if ( is any twice differentiable function then y(x, t) = 1/2[((x - ct) + ((x + ct)] satisfies this...
-
Presented below are income statements prepared on a LIFO and FIFO basis for Kenseth Company, which started operations on January 1, 2024. The company presently uses the LIFO method of pricing its...
-
Aspartame (below) is an artificial sweetener used in diet soft drinks and is marketed under many trade names, including Equal TM and Nutrasweet TM . In the body, aspartame is hydrolyzed to produce...
-
Draw a plausible mechanism for each of the following transformations: a. b. c. d. e. Pyridine CI
-
Ethyl trichloroacetate is significantly more reactive toward hydrolysis than ethyl acetate. Explain this observation.
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


