A saturated solution of KCl was prepared at 40.0C using 5.00 lb of water. How many grams
Question:
A saturated solution of KCl was prepared at 40.0°C using 5.00 lb of water. How many grams of KCl were required to prepare this solution?
Data from Problem 12.94
Use these data to plot solubility as a function of temperature for KCl and Li2SO4:
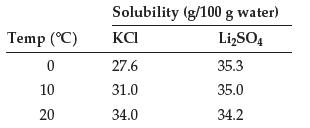
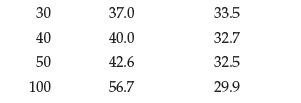
Using the plot, estimate the solubility of both compounds in water at 70°C.
How much of each compound can be dissolved in a beaker containing 75 g of water at 70°C?
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:




