A student forgets that the N in ammonia, NH 3 , has a lone pair as well
Question:
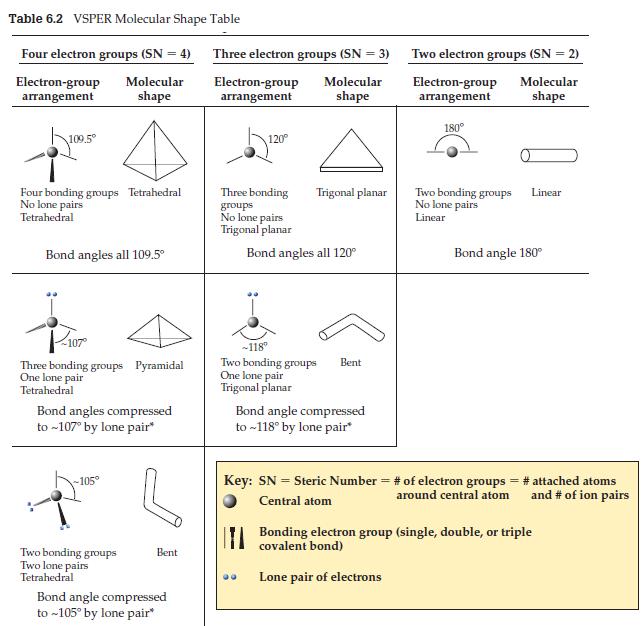
A student forgets that the N in ammonia, NH3, has a lone pair as well as its three single bonds. After checking Table 6.2, he mistakenly draws the molecule—three bonding groups, no lone pairs— as having a trigonal planar shape. If the ammonia molecule really were trigonal planar, how would the intermolecular forces differ from what they actually are?
Transcribed Image Text:
Table 6.2 VSPER Molecular Shape Table Four electron groups (SN = 4) Molecular shape Electron-group arrangement 109.5° Four bonding groups Tetrahedral No lone pairs Tetrahedral Bond angles all 109.5⁰ Three bonding groups Pyramidal One lone pair Tetrahedral Bond angles compressed to ~107° by lone pair* -105° Two bonding groups Two lone pairs Tetrahedral Bent Bond angle compressed to ~105° by lone pair* Three electron groups (SN = 3) Molecular shape Electron-group arrangement 120° Three bonding groups No lone pairs Trigonal planar Trigonal planar Bond angles all 120° -118° Two bonding groups One lone pair Trigonal planar Bent Bond angle compressed to ~118° by lone pair* Two electron groups (SN = 2) Electron-group Molecular shape arrangement 180° Two bonding groups No lone pairs Linear Linear Bond angle 180° Key: SN = Steric Number = # of electron groups = # attached atoms Central atom around central atom and # of ion pairs Bonding electron group (single, double, or triple covalent bond) Lone pair of electrons
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Explanation If ammonia molecule were trigonal planar then it would be a nonp...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
We left three possible situations out of Table 6.2: 1. Four electron groups (SN = 4): one bonding group and three lone pairs. 2. Three electron groups (SN = 3): one bonding group and two lone pairs....
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Problem 3.3 Minimize the functional J(x()) = f sx (s) x (s) ds, = 1 sr (s) i (s) ds, 0 subject to the endpoint conditions x (0) = 0 and x (1) = 1.
-
In cost analysis, what governs which costs are to be included in the study?
-
In February 2008, Ceramic Crucibles of America was notified by the state of Colorado that the state was investigating the company's Durango facility to determine if there were any violations of...
-
An increase in the required return _________ dividends.
-
A blending tank that provides nearly perfect mixing is connected to a downstream unit by a Long transfer pipe. The blending tank operates dynamically like a first-order process. The mixing...
-
Currently, all non-current assets, including Land and Buildings, Motor Vehicles and Furniture and Fixtures are reported in the Statement of Financial Position at historical cost. I would like to...
-
Explain the difference between electron-group geometry and molecular shape. How do you use electron-group geometry when deciding what shape a molecule has?
-
Draw the three-dimensional shape of methanol, CH 3 OH. Indicate the numeric value of all bond angles. Is this molecule polar? If so, draw the molecular dipole moment vector.
-
Intex Corporation is a multinational company with approximately 100 subsidiaries and divisions, referred to as reporting units. Each reporting unit operates autonomously and maintains its own...
-
MAT 152 Project 3: MLB Team Salaries The data set below is the total salary of each Major League Baseball (MLB) team salaries per team in 2016. Find the probabilities for normal distributions and...
-
deficit, surplusincreased, decreased$795, $1,975, $54,635, $35 6. Cash-flow statement Sam and Joan Wallingford have been married for two years. They have been trying to save but feel that there is...
-
Trudy bought the vacant lot adjacent to her house and planted a large garden there. The garden produces more vegetables than her family needs, and Trudy earns some extra cash by selling them at a...
-
Requirements Medical researchers once conducted experiments to determine whether Lisinopril is a drug that is effective in lowering systolic blood pressure of patients. Patients in a treatment group...
-
1. Balroop while looking for Gurjap walks 315m [N] toward the forest, then 133 m [28 S of E] through it, and finally finds him deep inside the forest after walking another 55 m [ 31 S of W]....
-
In Problems 1-2, find the maximum and minimum of the friction f over the closed and bounded set S. Use the methods of Section 12.8 to find the maximum and minimum on the interior of S; then use...
-
Identify Thank You mission, strategy and core competencies. Identify strategy changes that have taken place at Thank You since its founding in 2008. Your answer must in text references and must be...
-
Identify the reagents necessary to produce each of the following compounds via an aldol reaction. (a) (b) (c) (d) (e)
-
Using formaldehyde and acetaldehyde as your only sources of carbon atoms, show how you could make each of the following compounds. You may find it helpful to review acetal formation. (a) (b) (c) (d)...
-
Draw a mechanism for the following transformation: NaOH, heat
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...

Study smarter with the SolutionInn App


