Beaker (a) contains the pure compound called hydrogen peroxide (formula H 2 O 2 ). Beaker (b)
Question:
Beaker (a) contains the pure compound called hydrogen peroxide (formula H2O2). Beaker (b) contains the pure compound called water (H2O). Both compounds are made to undergo a change.
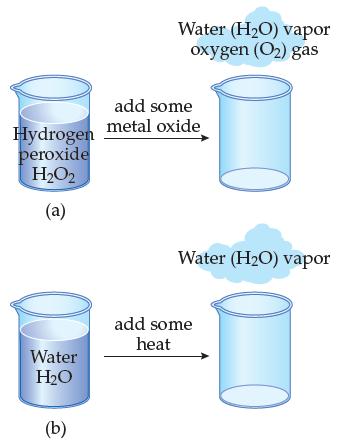
Which compound is undergoing a physical change, and which is undergoing a chemical change? Explain the reason for each.
Transcribed Image Text:
Hydrogen peroxide H₂O₂ (a) Water H₂O (b) Water (H₂O) vapor oxygen (O₂) gas add some metal oxide Water (H₂O) vapor add some heat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Hydrogen Peroxide H2O2 Change Decomposition into water vapor H2O and oxygen gas O2 by adding some ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Consider the following population regression model: y = Bo + Bx + Bx2 + 3x3 + u Suppose you want to test whether 0.532 = 83. The hypotheses are: Ho : 0.582 = 33 H : 0.532 #33 The correct expression...
-
American Corporation received a $400,000 low bid from a reputable manufacturer for the construction of special production equipment needed by American in an expansion program. Because its own plant...
-
Scotia bank operates in 50 countries and employs Canadians to staff its New Delhi office. What are the advantages of employing Canadians with roots in the host country? Would you use expatriate...
-
Assume there is a representative investor with constant relative risk aversion . Assume aggregate consumption C satisfies dC C = (X)dt + (X) dB for functions and , where X is the Markov process...
-
Mary Karston was hired by a popular fast-food restaurant as an order-taker and cashier. Shortly after taking the job, she was shocked to overhear an employee bragging to a friend about shortchanging...
-
You own a zero which pays annually and the yield is currently 8%. If the price of the bond is $495, what is the number of years to maturity? 9.14 years 17.9 years 8.96 years 7.22 years None of the...
-
What must Albert do to turn his Intense Facial Watching Hypothesis into a theory?
-
Consider the following two pieces of metal. Both consist of gold atoms (filled spheres) and silver atoms (open spheres). Which would be considered heterogeneous? Explain why. (a) (b)
-
Each of the following taxpayers received a state income tax refund in 2019. In all cases, the taxpayer has a filing status of married filing jointly. What amount of the refund is properly included in...
-
Perpetual Inventory Control Record Description: M & B Supreme Date Purchase Received Issued Sales Units Unit Cost June 1 Balance forward 3 $10.00 4 2 6 8 9 $10.50 9 12 32 3 6 2 4 15 6 10 $11.00 18 20...
-
A rectangular footing of size 4m by 5m is founded at 2m below ground level in a uniform deposit of saturated clay. The footing is designed to support a total vertical load of 8000 kN inclusive of the...
-
P6.2 At the start of Tom Stoppard's "Rosencrantz and Guildenstern are dead" 1, Rosencrantz finds a coin. Guildenstern watches as Rosencrantz repeatedly tosses the coin and every time it comes down...
-
For the data: 9 5 10 7 9 10 11 8 12 769 a) Compute the z-score for the raw score of 10 b) Find the raw score that corresponds to z=+1.22
-
(11%) Problem 7: After a bad thunderstorm, a loose power line comes to rest on a parked van. The van is insulated from the ground by its tires, and accumulates an electric charge of Q = 0.0012...
-
Order the 1H NMR signals of the following compounds by chemical-shift position (lowest to highest). Which one is the most upheld? The most downfield? (a) H3C-CH3 (b) H2C==CH2 (c) H3C-O-CH3 (d) (e)...
-
The overall reaction and equilibrium constant value for a hydrogenoxygen fuel cell at 298 K is 2H 2 (g) + O 2 (g) 2H 2 O(l) K = 1.28 10 83 a. Calculate E cell and G 8 at 298 K for the fuel cell...
-
The regions of + in a compound are the regions most likely to be attacked by an anion, such as hydroxide (HO - ). In the compound below, identify the two carbon atoms that are most likely to be...
-
Consider the three compounds shown below and then answer the questions that follow: a) Which two compounds are constitutional isomers? b) Which compound contains a nitrogen atom with trigonal...
-
Compound A is an alkene that was treated with ozone (followed by DMS) to yield only 4-heptanone. Identify the major product that is expected when compound A is treated with MCPBA followed by aqueous...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


