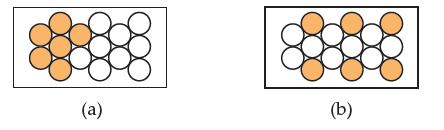
Consider the following two pieces of metal. Both consist of gold atoms (filled spheres) and silver atoms
Question:
Consider the following two pieces of metal. Both consist of gold atoms (filled spheres) and silver atoms (open spheres). Which would be considered heterogeneous? Explain why.
Transcribed Image Text:
(a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a Piece of metal a would be considered heterogeneous because it is made up of gold atoms and silver ...View the full answer

Answered By

Rohith Bellamkonda
I am studying in IIT Indore,the most prestigious institute of India.I love solving maths and enjoy coding
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Consider the following two risky asset worlds: Answer the following questions: a. Explain why the risk of any portfolio composed of (S) and (B) is less than weighted average risk of the two...
-
Dunkin Donuts What are all of Dunkin Donuts brands or product lines in this product mix? How does Dunkin Donuts promote itself? Starbucks What are all of Starbuck brands or product lines in this...
-
Consider the following two separate firms. One firm manufactures flexible packaging films for the snack, bakery, confectionery, and tobacco industries. Its manufacturing process has been quite stable...
-
For the curve defined by F(t) = (etcos(t), e* sin(t)) find the unit tangent vector, unit normal vector, normal acceleration, and tangential acceleration at 5A t 6 T N (5r) = 6 5T 6 aN ||
-
(a) What is the basic difference between the cost method and the par value method of accounting for treasury stock? (b) How will total stockholders equity differ, if at all, under the two methods?
-
A mild steel ring has a radius of 50 mm and a cross-sectional area of 400 mm. A current of 0.5 A flows in a coil wound uniformly around the ring and the flux produced is 0.1 mWb. If the relative...
-
Adopt the assumptions of Part (a) of Exercise
-
Laramie Corporations income statement is presented below: Sales ........ $40,000 Less variable costs .. -28,000 Contribution margin . $12,000 Less fixed costs ..... -8,000 Net Income ..... $ 4,000...
-
The CEO of Harding Media Inc. as asked you to help estimate its cost of common equity. You have obtained the following data: D 0 = $0.85; P0 = $22.00; and dividend growth rate = 6.00% (constant). The...
-
Beaker (a) contains the pure compound called hydrogen peroxide (formula H 2 O 2 ). Beaker (b) contains the pure compound called water (H 2 O). Both compounds are made to undergo a change. Which...
-
Which one of the following is a compound: ozone, 18-karat gold (made by melting gold and other metals together), NaCl, liquid nitrogen, iced tea?
-
Supply the IUPAC systematic name for each of the following ternary oxyacids. (a) HClO 2 (aq) (b) H 3 PO 4 (aq).
-
The following data are available for S&R company7 for its first month of operations: Direct materials Direct labor @P40/hr Job 101 Job 102 Job 103 P60,000 P90,000 P56,000 18,000 36,000 38,000...
-
1 2. Let A(x) = sin t + 1 dt, find A'(x) at x = 0, and 2 3. Evaluate the following definite integrals: 2 (a) (3x + 4x)dx 4 (b) xdx
-
A debt can be repaid with payments of $3912 today, $2436 in 2 years and $6770 in 5 years. What single payment will settle the debt 3 years from now if interest is 10.5% compounded quarterly?
-
With the PID/Freeze Frame/Snapshot data monitor function, input/output signal monitor items set in the start/stop control module can be selected and read out in real-time. Answer the following PID...
-
Which of the four global strategies (International, Multidomestic, Global-Standardization, or Transnational strategy) is 3M using? Is this the best strategy for it to use? Why or why not?
-
The 1H NMR spectrum of CH3COCH2C(CH3)3, 4,4-dimethyl-2-pentanone, taken at 300 MHz shows signals at the following positions: 307, 617, and 683 Hz downfield from tetramethylsilane (a) What are the...
-
Chicago Company sold merchandise to a customer for $1,500 cash in a state with a 6% sales tax rate. The total amount of cash collected from the customer was $558. $600. $642. $636. Nevada Company...
-
Identify the reagents necessary to achieve each of the following transformations: Br Br - " Br Br "Br
-
Determine the configuration for every chirality center in each of the following compounds. a. b. c. - - - - - - CH- - CH- II
-
For each of the following reactions predict the sign of G. If a prediction is not possible because the sign of G will be temperature dependent, describe how G will be affected by raising the...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


