Complete this table of ionic compounds. Give both old and new names where you can. Name Sodium
Question:
Complete this table of ionic compounds. Give both old and new names where you can.
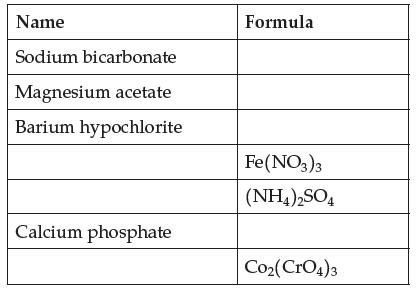
Transcribed Image Text:
Name Sodium bicarbonate Magnesium acetate Barium hypochlorite Calcium phosphate Formula Fe(NO3)3 (NH4)2SO4 Co₂(CrO4)3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Name Old name New name Sodium bicarbonate Baking soda NaHCO3 Magnesium acetate Epsom salt MgCH3COO2 ...View the full answer

Answered By

PRADEEP KUMAR BARAI
i love teaching currently i am working in corporate company before the job i was working as an home tutore for all physical science, maths and Electronics related subjects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Complete this table of ionic compounds. Give both old and new names where you can. Name Aluminum selenide Lithium oxide Ammonium iodide Formula AgNO3 CuSO4 KMnO4 Ca(ClO3)2
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Suppose you have the following training set, and fit a logistic regression classifier : ho(x) = g(00+011+0x2) O O O Which of the following are true? Check all that apply. a) Adding polynomial...
-
Although payroll records may vary in design, what types of employee data would be found in the payroll records of most manufacturing companies?
-
How do companies account for receivables that are factored?
-
What information must an enterprise report by geographic area? LO3
-
The December 31, 2021, unadjusted trial balance for the Wolkstein Drug Company is presented below. December 31 is the company?s year-end reporting date. The following year-end adjusting entries are...
-
Complete the table below for the missing variances. (Click the icon to view the table.) Calculate the variances and identify whether the variance is favorable (F) or unfavorable (U). (b) Total Direct...
-
Complete this table of molecular compounds. Name Nitrogen triiodide Bromine trifluoride Hydroiodic acid Formula CdTe Sil HI (as a pure gas) S4N4
-
Arrange in order of increasing ionic character: CsBr, KBr, PBr 3 , MgBr 2 .
-
Ralph placed a classified ad to sell his used SUV for $18,500. After 2 weeks, he didnt sell the SUV, and the newspaper suggested lowering the price 5%. What would the new price be if Ralph reduced it...
-
CASE 7.2 Oracle Corporation: Share-Based Compensation Effects/Statement of Shareholders' Equity A sales-based ranking of software companies provided by Yahoo! Finance on November 5, Year 8, places...
-
A manufacturer of ovens sells them for $1,450 each. The variable costs are $800 per unit. The manufacturer's factory has annual fixed costs of $1,735,000. a. Given the expected sales volume of 3,100...
-
1.1 Explain the vitality of a strategy on businesses like Dell. (15) 1.2 Critically discuss the underlying objectives Dell should follow when formulating its business strategy. (20) 1.3 Discuss the...
-
Our international business plan involves exporting a sustainable apparel brand from India to UK. We will be exploring this plan in further detail below: Product/ Service: Sustainable clothing line...
-
If X is a random variable with probability density function f given by: f (x) = 4x 4x 3 when 0 x 1 and 0 otherwise, compute the following quantities: (a) The cumulative distribution function F...
-
Find the point on the plane 2x + 4y + 3z = 12 that is closest to the origin. What is the minimum distance?
-
Show that the block upper triangular matrix A in Example 5 is invertible if and only if both A 11 and A 22 are invertible. Data from in Example 5 EXAMPLE 5 A matrix of the form A = [ A11 A12 0 A22 is...
-
Assume that a sealed vessel at constant pressure of 1 bar initially contains 2.00 mol of NO 2 (g). The system is allowed to equilibrate with respect to the reaction 2NO 2 (g) N 2 O 4 (g). The number...
-
When 1,3-dinitrobenzene is treated with nitric acid and sulfuric acid at elevated temperature, the product is 1,3,5-trinitrobenzene. Explain the regiochemical outcome of this reaction. In other...
-
Does chlorination of chlorobenzene require the use of a Lewis acid? Explain why or why not?
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


