Complete this table of ionic compounds. Give both old and new names where you can. Name Aluminum
Question:
Complete this table of ionic compounds. Give both old and new names where you can.
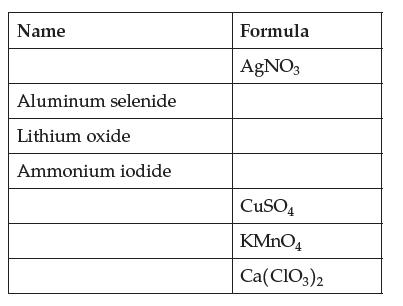
Transcribed Image Text:
Name Aluminum selenide Lithium oxide Ammonium iodide Formula AgNO3 CuSO4 KMnO4 Ca(ClO3)2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Name Old name New name AgNO3 Silver nitrate Silver nitrate Al2Se3 Aluminum selenide A...View the full answer

Answered By

Kainat Shabbir
i am an experienced qualified expert with a long record of success helping clients overcome specific difficulties in information technology, business and arts greatly increasing their confidence in these topics. i am providing professional services in following concerns research papers, term papers, dissertation writing, book reports, biography writing, proofreading, editing, article critique, book review, coursework, c++, java, bootstarp, database.
5.00+
184+ Reviews
255+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Complete this table of ionic compounds. Give both old and new names where you can. Name Sodium bicarbonate Magnesium acetate Barium hypochlorite Calcium phosphate Formula Fe(NO3)3 (NH4)2SO4 Co(CrO4)3
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Presented here are summarized data from the balance sheets and income statements of Wiper Inc.: WIPER INC. Condensed Balance Sheets December 31, 2020, 2019, 2018 (in millions) 2020 2019 Current...
-
In a payroll system, what purpose is served by a labor time record? How is the information that is recorded on labor time records used?
-
From the partial work sheet for Adams' Shoe Shine on the next page, prepare an income statement. Adams Shoe Shine Work Sheet (Partial) For Month Ended June 30, 20- NCOME STATEMENT ALANCE SHEET...
-
Under what conditions should a company disclose the amount of sales from a major customer? LO3
-
The Gallup Organization regularly surveys adult Americans regarding their commute time to work. In addition, they administer a Well-Being Survey. According to the Gallup Organization, The...
-
e 1: Question 8 (6 points) 2 Joanna is making a financial plan for her retirement. Today is her 35th birthday, and she receives $150,000 from her grandmother's will. She plans to retire 25 years from...
-
Arrange in order of increasing ionic character: CsBr, KBr, PBr 3 , MgBr 2 .
-
Iodine atoms in I2 should have a (a) 1 charge (b) charge (c) + charge (d) No charge
-
A chemical plant requires 106 L/day of a solution. Three sources are available at different prices and supply rates. Each source also has a different concentration of an impurity that must be kept...
-
From Hoffman, what are the symptoms of autism and ADHD? From the Mayo Clinic, what are the causes and risk factors for autism and ADHD? What are treatment options for these disorders? Hofmann, S. G....
-
Brooks, a participant in the Zappa retirement plan, has requested a second plan loan. His vested account balance is $80,000. Brooks borrowed $27,000 eight months ago and still owes $18,000 on that...
-
Suppose that Angelina and Brad own the only two professional photography stores in town. Each must choose between a low price and a high price for senior photo packages. The annual economic profit...
-
based on the article How Chili's Is Prepping for Tough Times, Starting With the Fries by Heather Haddon. What is corporate social responsibility, and what is one way that Chili's can better pursue...
-
QUESTION 2 (20 marks) CLO 5 a. Explain what the following ratios indicate to a firm: (i) Acid Test Ratio (ii) Return on Capital Employed (ROCE) (iii) Debtors Collection Period (iv) Working Capital (2...
-
A rectangular box, whose edges are parallel to the coordinate axes, is inscribed in the ellipsoid 96x2 + 4y2 + 4z2 = 36. What is the greatest possible volume for such a box?
-
Vectors are drawn from the center of a regular n-sided polygon in the plane to the vertices of the polygon. Show that the sum of the vectors is zero.
-
The following compound has four aromatic rings. Rank them in terms of increasing reactivity toward electrophilic aromatic substitution.
-
For each compound below, identify which position(s) is/are most likely to undergo an electrophilic aromatic substitution reaction. (a) (b) (c) (d) (e) (f) (g) (h) (i) CH3 NO2 O,N. CH3 O,N NO2
-
Predict the product(s) for each of the following reactions: (a) (b) (c) OH HNO, H,SO, OMe Br Br2 FeBr3
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


