Complete this table of molecular compounds. Name Nitrogen triiodide Bromine trifluoride Hydroiodic acid Formula CdTe Sil HI
Question:
Complete this table of molecular compounds.
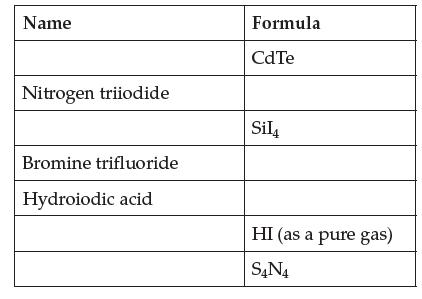
Transcribed Image Text:
Name Nitrogen triiodide Bromine trifluoride Hydroiodic acid Formula CdTe Sil HI (as a pure gas) S4N4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Name Formula Ethanol C2H6O Acetic acid CH3COOH Glucose C6H12O6 Sucrose C12H2...View the full answer

Answered By

Felix Onchweri
I have enough knowledge to handle different assignments and projects in the computing world. Besides, I can handle essays in different fields such as business and history. I can also handle both short and long research issues as per the requirements of the client. I believe in early delivery of orders so that the client has enough time to go through the work before submitting it. Am indeed the best option that any client that can think about.
4.50+
5+ Reviews
19+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
Diffusion in a gas is the random motion of particles involved in the net movement of a substance from an area of high concentration to an area of low concentration. The process of diffusion also...
-
Write the molecular and structural formulas for the compounds represented by the following molecular models: (a) (b) (c) (d) P F F F
-
To be more engaged in your community, think of any issues that the youth today are facing, then find books, or studies that will help you in supporting your topic. Write your own observation/s on the...
-
What are the sources for posting direct labor cost to (a) Individual jobs in the job cost ledger and (b) The work in process account in the general ledger?
-
How do firms report assets on the balance sheet?
-
Under what conditions must an enterprise provide information about geographic areas? LO3
-
After the Supreme Courts 1995 decision in Adarand v. Pea what requirements did an affirmative action program have to meet to be constitutional?
-
\Blossom Street Inc. makes unfinished bookcases that it sells for $ 58. Production costs are $ 38 variable and $ 10 fixed. Because it has unused capacity, Blossom Street is considering finishing the...
-
Complete this table of molecular compounds. Name Iodine monochloride Bromine trichloride Diboron hexahydride Formula XeF4 XeF NO S402
-
Complete this table of ionic compounds. Give both old and new names where you can. Name Sodium bicarbonate Magnesium acetate Barium hypochlorite Calcium phosphate Formula Fe(NO3)3 (NH4)2SO4 Co(CrO4)3
-
Indicate the concentration of each ion or molecule present in the following solutions: (a) 0.25 M NaNO3 (b) 1.3 10-2 MgSO4 (c) 0.0150 M C6H12O6 (d) A mixture of 45.0 mL of 0.272 M NaCl and 65.0 mL...
-
MTB Surfboards has a P / E of 2 0 . The discount rate for this firm is 3 0 percent. They had earnings of $ 2 , 0 0 0 , 0 0 0 and 1 0 0 , 0 0 0 shares of common stock outstanding. What should be the...
-
Question 4 (20 marks) Laboratory 4: Superposition Theorem Objectives: 1. Understand the principles of a Superposition Theorem 2. Determine the characteristics of a Superposition Theorem...
-
2 Ursala, Inc., has a target debt-equity ratio of .65. Its WACC is 10.4 percent, and the tax rate is 23 percent. a. If the company's cost of equity is 14 percent, what is its pretax cost of debt? b....
-
Thinking about Nike's corporate practices, discuss your approach to starting a company that outsourced labor in order to reduce manufacturing costs. What decisions would you make to combine...
-
Owen Properties recently purchased a building in a community that is eligible for participation in the National Flood Insurance Program (NFIP). Under the regular program of the NFIP, the maximum...
-
Find the point on the paraboloid z = x2 + y2 that is closest to (1, 2.0). What is the minimum distance?
-
Do the three planes x + 2x + x 3 = 4, X X 3 = 1, and x + 3x = 0 have at least one common point of intersection? Explain.
-
When ethoxybenzene is treated with a mixture of nitric acid and sulfuric acid, two products are obtained each of which has the molecular formula C 8 H 9 NO 3 . (a) Draw the structure of each product....
-
The pressure dependence of G is quite different for gases and condensed phases. Calculate G m for the processes (C, solid, graphite, 1 bar, 298.15 K) (C, solid, graphite, 325 bar, 298.15 K) and (He,...
-
Many biological macromolecules undergo a transition called denaturation. Denaturation is a process whereby a structured, biologically active molecule, called the native form, unfolds or becomes...
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


