Consider the basic hydrolysis (reaction with aqueous base) of (CH3) 3 CBr. The rate law is first
Question:
Consider the basic hydrolysis (reaction with aqueous base) of (CH3)3CBr.
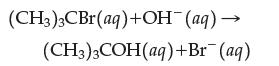
The rate law is first order with respect to (CH3)3CBr and zero order with respect to OH–. What does this imply about the mechanism of this reaction?
Transcribed Image Text:
(CH3)3CBr(aq) +OH¯(aq) → (CH3)3COH(aq) + Br (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The fact that the rate law for the basic hydrolysis of CH33CBr is first order with respect to CH33CB...View the full answer

Answered By

Lisper Wanja
I am an experienced and highly motivated writer with a passion for the skills listed. I have a proven track record of my expertise and my aim is to deliver quality, well-detailed and plagiarism free projects. My genuine passion for writing combined with my ongoing professional development through school and research makes me an ideal candidate within for any assignment.
4.90+
233+ Reviews
388+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Consider a teenager who evaluates whether she should engage in sexual activity with her partner of the opposite sex. She thinks ahead and expects to have a present discounted level of life-time...
-
The decomposition reaction of N2O5 in carbon tetrachloride is 2 N2O5 -- 4 NO2 + O2. The rate law is first order in N2O5. At the rate constant is 4.82 10-3 s-1. (a) Write the rate law for the...
-
Consider a simple time series model where the explanatory variable has classical measurement error: where ut has zero mean and is uncorrelated with x*t, and et. We observe yt and xt only. Assume that...
-
In which section do you create VLAN on Cisco WLC ? ? Layer 3 3 Section Layer 2 2 Section Security Section RF Section
-
Briefly explain what is meant by transaction analysis. What are the two steps in transaction analysis?
-
The following table gives data on imports, GDP, and the Consumer Price Index (CPI) for the United States over the period 19752005. You are asked to consider the following model: ln Importst =...
-
New Colony Corporation (a U.S. company) made a sale to a foreign customer on September 15, 2009, for 100,000 foreign currency units (FCU). It received payment on October 15, 2009. The following...
-
Chip, I know the interviews took a long time, but they were worth it, Anna says defensively as Chip enters her office with a worried look on his face. Im sure of that, Chip says. You really made a...
-
The proper real estate industry term for the document that escrow transmits to a buyer, giving the right to the seller to cancel the escrow if the buyer does not take the contractual actions...
-
Consider the decomposition of ozone (O 3 ) to oxygen (O 2 ). The rate law for this reaction is: Rate = k[O 3 ] 2 /[O 2 ]. How is the rate of this reaction affected by the concentration of oxygen?...
-
Consider the decomposition reaction of ozone into oxygen: 2O 3 (g) 3O 2 (g) Suppose the mechanism for this reaction is just the collision between two ozone molecules, as shown in the following...
-
1 Identify three factors that could underlie the store managers perceptions of racially motivated behaviour by his white colleagues. V2 Is there a link between perceptions of racism in interracial...
-
2. If w = x + y z + sint and x + y = t, find Iw dw Iw a. b. c. av z x, z aw Iw aw d. e. f. az at at y, t x, Z y, z
-
Global Training Solutions (GTS) was founded 40 years ago by an educator/consultant/trainer to provide training to business employees, consulting services to businesses and school systems, and...
-
Consider the following open-loop transfer function: K(s+2a) G(s)H(s)= where K,a>0 s(s-a) The frequency when the phase is -180 degrees is: rad/s The magnitude of the open loop transfer function when...
-
Complete Exhibit 5 (this should be cost per cup and in CA$, do not convert). The exhibit is missing a line for Milk & Sugar within factory overhead section EXHIBIT 5: COSTING CHART Direct Cost Coffee...
-
Why is the tension negative in the net force x-component? The tension points right, so shouldn't it be positive? I saw elsewhere that the equation has negative T. Why? Chrome File Edit View History...
-
Suppose that 10,000 men and 10,000 women settle on an island in the Pacific that has been opened to development. Suppose also that a medical study of the settlers finds that 200 of the men are...
-
Nitrogen monoxide reacts with hydrogen as follows: 2NO(g)+ H2(g) N2O(g) + H2O(g) The rate law is [H2]/ t = k[NO]2[H2], where k is 1.10 107 L2/(mol2s) at 826oC. A vessel contains NO and H2 at...
-
Pulling gs. Suppose again you are the astronaut in Problem 9. When most people are subjected to an acceleration greater than about 5 g, they will usually become unconscious (black out). Will you be...
-
Consider a string with one end tied to a tall ceiling and the other end hanging freely. Explain why the tension at the bottom of the string is smaller than the tension at the top.
-
Draw a qualitative plot of the total force acting on the ball in Figure 3.15 (page 68) as a function of time. Begin your plot while the ball is still in the throwers hand and end it after the ball...
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


