Does the following reaction-energy profile represent an endothermic or exothermic reaction in the forward direction? In the
Question:
Does the following reaction-energy profile represent an endothermic or exothermic reaction in the forward direction? In the reverse direction?
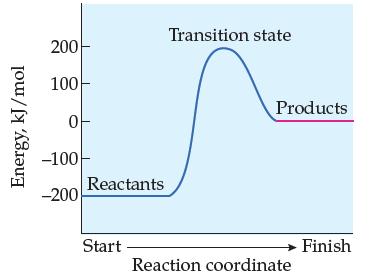
Transcribed Image Text:
Energy, kJ/mol 200 100- 0 -100 -200 Reactants Start Transition state Products Reaction coordinate Finish
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The reactionenergy profile represents an endothermic reaction in the forward direction and an exoth...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
What is the activation energy for the reverse reaction in Problem 13.95? Data from Problem 13.95 Does the following reaction-energy profile represent an endothermic or exothermic reaction in the...
-
What is the activation energy for the forward reaction in Problem 13.95? Data from Problem 13.95 Does the following reaction-energy profile represent an endothermic or exothermic reaction in the...
-
What is E forward rxn for the reaction in Problem 13.95? What is E reverse rxn ? Data from Problem 13.95 Does the following reaction-energy profile represent an endothermic or exothermic reaction in...
-
True or False? Azure files can be accessed from anywhere in the world using a URL that points to the file. True False
-
Inferring Year-End Adjustments, Computing Earnings per Share and Net Profit Margin, and Recording Closing Entries Ramirez Company is completing the information processing cycle at its fiscal...
-
Are there factors besides learning that can help reduce costs as volume increases?
-
What arc three potentially significant differences between IFRS and U.S. GAAP with respect to the recognition or measurement of assets? LO4
-
A study reported in the Journal of Small Business Management concluded that self-employed individuals do not experience higher job satisfaction than individuals who are not self-employed. In this...
-
18. A company has sales of $30,000, $6,000 cost of goods sold, expenses of $4,000, an interest expense of $2,000 and a tax rate of 5%. a) what is its net income? b) what is its earning per share if...
-
From the following reaction-energy profiles, determine whether reactions A and B are exothermic or endothermic in the forward direction: Energy, kJ/mol 300 200 100 0 -100 -200 B Reactants Start...
-
In a kinetic study of the reaction the following rate data were obtained. Write a rate law complete with proper values for the orders. What is the overall order of the reaction? 2 C10(aq)+ 2 OH (aq) ...
-
What is the output produced from the following statements? System.out.println("name\tage\theight"); System.out.println("Archie\t17\t5'9\""); System.out.println("Betty\t17\t5'6\"");...
-
X 18. State the amplitude and period of: y = -4cos Graph one cycle of the function. 4 1 19. State the amplitude and period of: y = -sin(4x) Graph one cycle of the function. 4
-
Explain ways in which an organisation may overcome security vulnerabilities and issues?
-
A nonpipelined system takes 300ns to process a task. The same task can be processed in a 4-stage pipeline with a clock cycle of 50ns. Determine the speedup ratio of the pipeline for 400 tasks. What...
-
Within an orthodontic practice that I work in, insufficient patient care and poor time management are the most significant issues in the office. Beginning with the receptionists, scheduling...
-
How has the decision been improved with more of a focus on financial information? Why would it have been a better decision? How could you have included more financial information and where might it...
-
Let A be an n n symmetric negative definite matrix. (a) What will the sign of det(A) be if n is even? If n is odd? (b) Show that the leading principal submatrices of A are negative definite. (c)...
-
A sprinkler head malfunctions at midfield in an NFL football field. The puddle of water forms a circular pattern around the sprinkler head with a radius in yards that grows as a function of time, in...
-
A baseball is thrown with an initial velocity of magnitude v 0 at an angle of 60 with respect to the horizontal (x) direction. At the same time, a second ball is thrown with the same initial speed at...
-
A high jumper can run horizontally with a top speed of 10.0 m/s. (a) If he can convert this velocity to the vertical direction when he leaves the ground, what is the theoretical limit on the height...
-
A bullet is fired from a rifle with speed v 0 at an angle θ with respect to the horizontal axis (Fig. P4.32) from a cliff that is a height h above the ground below. (a) Calculate the...
-
The star Mira is 1.2 times the mass of the Sun and about 10,000 times more luminous than the Sun. Would Mira fit into the table above? Why or why not?
-
Which of the following was one of the most valuable benefits a company received as a sponsor of NHL games?
-
Cinder Inc. is a Canadian-controlled private corporation based in your province. The company operates a wholesale business. The following information is provided for its year ended May 31, 2023: Net...

Study smarter with the SolutionInn App


