For each reaction, write the K eq expression. Then decide which of the following equilibrium constants goes
Question:
For each reaction, write the Keq expression.
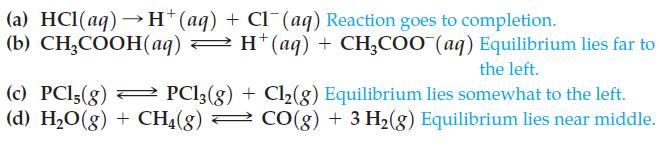
Then decide which of the following equilibrium constants goes with each reaction:
![]()
Transcribed Image Text:
(a) HCl(aq)→ H+(aq) + Cl¯(aq) Reaction goes to completion. (b) CH₂COOH(aq) → H* (aq) + CH₂COO (aq) Equilibrium lies far to the left. = (c) PC15(g) PC13(g) + Cl₂(g) Equilibrium lies somewhat to the left. (d) H₂O(g) + CH4(g) → CO(g) + 3 H₂(g) Equilibrium lies near middle.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a b c...View the full answer

Answered By

Anthony Ngatia
I have three academic degrees i.e bachelors degree in Education(English & Literature),bachelors degree in business administration(entrepreneurship option),and masters degree in business administration(strategic management) in addition to a diploma in business management.I have spent much of my life in the academia where I have taught at high school,middle level colleges level and at university level.I have been an active academic essays writer since 2011 where I have worked with some of the most reputable essay companies based in Europe and in the US.I have over the years perfected my academic writing skills as a result of tackling numerous different assignments.I do not plagiarize and I maintain competitive quality in all the assignments that I handle.I am driven by strong work ethics and a firm conviction that I should "Do Unto others as I would Like them to do to me".
4.80+
76+ Reviews
152+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
The solution containing no added KNO3 for Figure 7-1 contains 5.0 mM Fe(NO3)3, 5.0 M NaSCN, and 15 mM HNO3. We will use Davies activity coefficients to find the concentrations of all species in the...
-
1. Explain the use of strategic option in valuation. Explain how strategic options are often abused in valuation. 2. Explain the APV approach and how it is used in enterprise valuation. 3. Explain...
-
The following equilibrium constants were determined at 1123 K: Write the equilibrium constant expression KP, and calculate the equilibrium constant at 1123 K for C(s) + CO2(g)--2CO(g) CO(g) + Cl2(g)...
-
Which one of the following statements related to investigations of workplace harassment complaints under Ontario's OHSA is true? a) As a rule, such investigations should be completed with 120 days of...
-
Preparing the Investing and Financing Sections of the Statement of Cash Flows Starwood Hotels & Resorts Worldwide, Inc., is one of the worlds largest hotel and leisure companies. It conducts business...
-
The demand for roses. The following table gives quarterly data on these variables: Y = quantity of roses sold, dozens X 2 = average wholesale price of roses, $/dozen X 3 = average wholesale price of...
-
In what way is the accounting for a foreign currency borrowing more complicated than the account ing for a foreign currency account payable? LO9
-
In the transformer shown in Figure P33.47, the load resistor is 50.0 Ω. The turns ratio N1:N2 is 5:2, and the source voltage is 80.0 V (rms). If a voltmeter across the load measures 25.0 V...
-
Viking Corp. uses a standard cost system to account for the costs of its one products. Material standards are 13 pounds of material at $1.30 per pound and 3 hours of labor at a standard wage rate of...
-
The equilibrium constant expression K eq for chemical reaction can be written as k f /k r because: (a) The ks are constants. (b) At equilibrium, k f [reactants] = k r [products], (the forward and...
-
Write the equilibrium constant expression for the reaction H 2 (g) + I 2 (g) 2HI(g)
-
Give conditions on the function \(h\) so that the measure \(\mathbb{Q}\) defined on \(\mathcal{F}_{T}\) as \(\mathbb{Q}=h\left(W_{T} ight) \mathbb{P}\) is a probability equivalent to \(\mathbb{P}\)....
-
The transmitted energy expands out into space as it propagates at 3 GHz between the transmitter and the receiver over 30 km distance. Calculate the free space loss using a suitable formula and any...
-
What is the company featured in this episode of Undercover Boss? List 3 good professional activities that the CEO/president learned about their company by going undercover? List areas of the...
-
Assume there is a national lottery in the winning ticket is worth $10 million one winning ticket will be selected if there are 225 million tickets sold. What is the chance that a buyer of one ticket...
-
Description: Reference: Basu Thakur. (2015). PostcolonialTheory and Avatar (pp. 85-150,157-172). Bloomsbury PublishingUSAPre-Peer Paper Review for the Postcolonial Application Paper 1: Collecting...
-
NOT ASKING THE ACTUAL SHEAR STRESS. Please READ! Derive the shear stress distributed equation over the cross-section. Derive the equation and plot. 15 15 30 15 15 120 -90 20 0.5 m 72 kN 20 20 40 40...
-
If a, b, and c are floating - point numbers, then fl(fl(a + b) + c) = fl(a + fl(b + c)) In the case of a true statement, explain or prove your answer. In the case of a false statement, give an...
-
Which of the followingcarbocations is the least stable? CH3CH2 . CH3CHCH3 CH3 I . CH3C0 T CH3 IV. V. CH3 CH3CCH2 CH3
-
A proposed design for a part of a seawall consists of a rectangular solid weighing 3840 lb with dimensions of 8.00 ft by 4.00 ft by 2.00 ft. The 8.00-ft side is to be vertical. Will this object float...
-
A platform is being designed to support some water pollution testing equipment. As shown in Fig. 5.31, its base is 36.00 in wide, 48.00 in long, and 12.00 in high. The entire system weighs 130 lb,...
-
A block of wood with a specific weight of 32 lb/ft 3 is 6 by 6 by 12 in. If it is placed in oil (sg = 0.90) with the 6 by 12-in surface parallel to the surface of the oil, would it be stable?
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


