In the following molecule, atoms X and Y are from the same period, meaning they are approximately
Question:
In the following molecule, atoms X and Y are from the same period, meaning they are approximately the same size. The difference in the size of the spheres indicates a difference in the amount of time the shared electrons spend near each atom. Which atom is more electronegative, and how do you know?
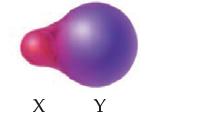
Transcribed Image Text:
X Y
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
y to attract electrons and atoms with higher electronegativity tend to attract electrons more strong...View the full answer

Answered By

AJIN kuriakose
I have completed B.Tech in Electrical Engineering & Masters in Power & Control From one of the best universities in India. I got the 99.05 percentile in the Gate Electrical Engineering Exam. I can Help students solving assignments in Electrical subjects like Power Electronics, Control system, Analog, Network Theory & Engineering Mathematics. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam.
Get guidance and the opportunity to learn from experienced...
I can provide tuition for Electrical engineering subjects (Power Electronics, Digital electronics, Network Theory, Control System & Engineering Mathematics). The toughest subject of Electrical engineering can be made simple in online classes...
I can also solve it.
1 .I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject.
Contact me in solution inn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advise related to tutoring and how it works here.thank you.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
You are interviewing a candidate for a position at a call center. You need someone polite, courteous, patient, and dependable. The candidate you are talking to seems nice. But how do you know who is...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
? ?? ? Prepare Company 1's COMPLETE CASH FLOW STATEMENT for 2016 (that includes CFO, CFI, CFF) using INDIRECT approach for CFO part. Explain how to treat for accounting purposes occupation of...
-
A company may select an inventory costing method from a number of commonly used procedures. Briefly, how would you describe each of the following methods? a. First-in, first-out b. Last-in, first-out...
-
Dr. Kristine Cohen opened a medical practice specializing in physical therapy. During the first month of operation (July), the business, titled Dr. Kristine Cohen, Professional Corporation (PC),...
-
Many companies make annual reports available on their corporate Internet home page. Annual reports also can be accessed through the SECs EDGAR system at www.sec.gov (under Forms, search for ARS or...
-
The comparative statements of Villa Tool Company are presented below and on the shown below. Instructions Compute the following ratios for 2012. (Weighted-average ordinary shares in 2012 were 57,000,...
-
Andrew Industries purchased $165,000 of raw materials on account during the month of March. The beginning Raw Materials Inventory balance was $22,000, and the materials used to complete jobs during...
-
Chemists sometimes think of molecules with polar covalent bonds as being part covalent and part ionic. How can a bond be both covalent and ionic?
-
Which molecule has bonds that are the most polar covalent? (a) H 2 (b) CO (c) H 2 S (d) H 2 O
-
A sample of 20 financial analysts was asked to provide forecasts of earnings per share of a corporation for next year. The results are summarized in the following table: Forecast ($ per share) Number...
-
f. The coordinates of two points A and B are (1, 2) and (5,7) respectively. Find the equation and slope of the line AB. g. Find the rate of change of the area of a circle w.r.t its radius r when r =...
-
1. Sketch the anticipated pattern of cracks on the beam structure shown below. Assume that the structure is adequately reinforced for the load shown, and that the loads are large enough to cause...
-
Estimate the hydrogen consumption required to completely remove the sulfur from a hydrotreater feedstock and to reduce the nitrogen content of the product to 15 ppm by weight. The 48.5 API naphtha...
-
2. Consider the following kinds of information, and suggest the most appropriate data type to store or represent each: Information Suggested Data Type String A person's name A person's age in years A...
-
Steam at 32 MPa, 520C enters the first stage of a supercritical reheat cycle including three turbine stages. Steam exiting the first-stage turbine at pressure p is reheated at constant pressure to...
-
If the temperature of a plate at the point (x, y) is T(x, y) = 10 + x2 - y2, find the path a heat-seeking particle (which always moves in the direction of greatest increase in temperature) would...
-
What are the principal alloying elements in SAE 4340 steel?
-
At what temperature does the slope of the z versus P curve as P 0 have its maximum value for a van der Waals gas? What is the value of the maximum slope?
-
Calculate the density of O 2 (g) at 480. K and 280. bar using the ideal gas and the van der Waals equations of state. Use a numerical equation solver to solve the van der Waals equation for V m or...
-
Show that T = 1 + T ( In z/T) P , and that Pk = 1 P( In z/T) T.
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


