Suppose the first step in the reaction of Problem 13.155 was the rate-determining step. Would the rate
Question:
Suppose the first step in the reaction of Problem 13.155 was the rate-determining step. Would the rate law be k[Cl2][CHCl3], k[Cl2], k[CHCl3], or k[Cl2]2? Explain.
Data from Problem 13.155
Write the overall balanced chemical equation that goes along with the mechanism:
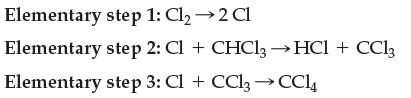
Transcribed Image Text:
Elementary step 1: Cl₂ → 2 Cl Elementary step 2: Cl + CHCl3→ HCl + CC13 Elementary step 3: Cl + CCl₂ → CC14
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
If the first step in the reaction of Problem 13155 was the ratedetermining step the rate law woul...View the full answer

Answered By

Ankur Gupta
I have a degree in finance from a well-renowned university and I have been working in the financial industry for over 10 years now. I have a lot of experience in financial management, and I have been teaching financial management courses at the university level for the past 5 years. I am extremely passionate about helping students learn and understand financial management, and I firmly believe that I have the necessary skills and knowledge to effectively tutor students in this subject.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Write the overall balanced chemical equation that goes along with the mechanism: Elementary step 1: Cl 2 Cl Elementary step 2: Cl + CHCl3 HCl + CC13 Elementary step 3: Cl + CCl CC14
-
Suppose the experimental rate law for the reaction in Practice Problem 13.24 is: Rate = k[Y 2 ] 2 Use the step postulated in Practice Problem 13.24 as the first step in a possibly correct mechanism...
-
The object is to study the kinetics of the reaction between peroxodisulfate and iodide ions. The I 3 formed in reaction (a) is actually a complex of iodine, I 2 and iodide ion, I. Thiosulfate ion, S...
-
A ball, which we can treat as a point charge, has a charge of +Q. This ball is 50 cm away from a ball of charge-100, which is fixed in position. The +Q ball is 30 cm vertically below, and 40 cm...
-
Analyzing the Effects of Transactions in T-Accounts Granger Service Company, Inc., was organized by Ted Granger and five other investors. The following activities occurred during the year: a....
-
Refer to Example 7.4. For this problem the correlation matrix is as follows: a. Since the zero-order correlations are very high, there must be serious multicollinearity. Comment. b. Would you drop...
-
Assuming that MNC entered into no forward contract, how much foreign exchange gain or loss should it report on its 2009 income statement with regard to this transaction? LO9 a. $5,000 gain. b. $3,000...
-
You are given the following data on two companies, M and N (figures are millions): Required: a. Which company has the higher profit margin? b. Which company has the higher investment turnover? c....
-
Exercise 1 7 - 1 1 ( Algo ) Components of pension expense; journal entries [ L 0 1 7 - 6 , 1 7 - 7 ] Pension data for Barry Financial Services Incorporated include the following: Discount rate, 7 % (...
-
The process to make ammonia (NH 3 ) gas is called the Haber process. The chemical reaction is shown below. To make it go fast enough tobe useful, it has to be run at high temperatures (400 C) and...
-
Suppose a postulated mechanism does generate the experimental rate law, and when the elementary steps are added together, the balanced equation for the overall reaction is generated. What can you say...
-
Refer to the Hunter Valley Snow Park Lodge expansion project in Short Exercise S26-4. Calculate the ARR. Round to two decimal places. Refer to Short Exercise S26-4, Consider how Hunter Valley Snow...
-
The figure shows a turbine-driven pump that provides water, at high pressure, to a tank located 25-m higher than the pump. Steady-state operating data for the turbine and the pump are labelled on the...
-
Step 1 Step 2 1. Sketch what step 4 and then step 5 would look like. Step 4 Step S 2. How many black triangles are in each step? Step 1 black A = | Step 2 = 4 black A's step 3 = 13 black D's 3. What...
-
The pressure cooker pictured here consists of a light pressure vessel with a heavy lid of weight W. When the lid is secured, the vessel is filled with a hot pressurized gas of pressure p. After some...
-
5) A large group of students took a test in Finite Math where the grades had a mean of 72 and a standard deviation of 4. Assume that the distribution of these grades is approximated by a normal...
-
Q9 (5 points) According to Dr. Henry Mintzberg, a noted management scholar from McGill University in Montreal, PQ, "business organizations perform only two activities of consequence." What are these...
-
Let A be a matrix with eigenvalues λ1. . . . . . λn and let λ be an eigenvalue of A + E. Let X be a matrix that diagonalizes A and let C = X-1EX. Prove (a) For...
-
On October 1, 2021, Adoll Company acquired 2,600 shares of its $1 par value stock for $38 per share and held these shares in treasury. On March 1, 2023, Adoll resold all the treasury shares for $34...
-
Water at 10C is flowing at 0.075 m 3 /s. Calculate the weight flow rate and the mass flow rate.
-
Oil for a hydraulic system (sg = 0.90) is flowing at 2 .35 10 -3 m 3 /s . Calculate the weight flow rate and mass flow rate.
-
After the refrigerant from Problem 6.31 flashes into a vapor, its specific weight is 12.50 N/m 3 . If the weight flow rate remains at 28.5 N/h, compute the volume flow rate. In Problem A liquid...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


