Write the overall balanced chemical equation that goes along with the mechanism: Elementary step 1: Cl 2
Question:
Write the overall balanced chemical equation that goes along with the mechanism:
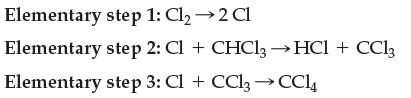
Transcribed Image Text:
Elementary step 1: Cl₂ →2 Cl Elementary step 2: Cl + CHCl3→ HCl + CC13 Elementary step 3: Cl + CCl₂ → CC14
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
The overall balanced chemical equation that goes along with the mechanism in the image you sent is C...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
3. Calculate and Explain, how much the action described has added to GDP: A company sells 50 computers at a retail price of $1000 apiece and 100 software packages at a retail price of $50 apiece to...
-
Suppose the first step in the reaction of Problem 13.155 was the rate-determining step. Would the rate law be k[Cl 2 ][CHCl 3 ], k[Cl 2 ], k[CHCl 3 ], or k[Cl 2 ] 2 ? Explain. Data from Problem...
-
The discovery of hafnium, element number 72, provided a controversial episode in chemistry. G. Urbain, a French chemist, claimed in 1911 to have isolated an element number 72 from a sample of rare...
-
11. What is the Specific Gravity of Zinc ? 12. The barometric pressure for the day is 14.7 psi. The hangar shop air tank gage reads 120 psi. What is the absolute pressure inside the shop air tank? =...
-
Recording Investing and Financing Activities Refer to E2-5. Required: 1. For each of the events (a) through (f) in E2-5, prepare journal entries, checking that debits equal credits. 2. Explain your...
-
Every year the U.S. Census Bureau releases the Current Population Report based on a survey of 50,000 households. The goal of this report is to learn the demographic characteristics, such as income,...
-
Assuming that MNC entered into a forward contract to sell 10 million South Korean won on December 1, 2009, as a fair value hedge of a foreign currency receivable, what is the net impact on its net...
-
Below is a brief description of a design-&-build power plant project. The top (i.e. northern) half of the plant plot contains the evaporation pond and tanks for storage of fuel. The...
-
A payday loan company charges 9 percent interest for a two - week period. What would be the annual interest rate from that company?
-
The process to make ammonia (NH 3 ) gas is called the Haber process. The chemical reaction is shown below. To make it go fast enough tobe useful, it has to be run at high temperatures (400 C) and...
-
Suppose a postulated mechanism does generate the experimental rate law, and when the elementary steps are added together, the balanced equation for the overall reaction is generated. What can you say...
-
Under communism, religion was frowned on in the countries of Eastern Europe. Do these countries remain less religious than the countries of Western Europe? Use the WVS variable RELPERSON in a t -test...
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28W / (m ^ 2) ."C. The thermal conductivity of the solid...
-
Which of the following best demonstrates the Six Cs of Communication, "you" approach, and positive emphasis? Question 1 4 options: It will be February 1 0 before you will receive your materials. It...
-
please answer all the questionss.,.within 30 minutes. make sure the explanation and reasons are explained in very detailed manner as in why the chosen option is right and why other options are wrong....
-
1) A net force of 20 N is applied to the right on an object. If the acceleration of the object is 2.5 m/sec, a) What is the mass of the object? (8 kg) b) What is the weight of the object? (78.4 N) c)...
-
BO Corp. has a $2,500 capital budget, and has access to the following 5 independent projects. In all these 5 projects, cash outflows occur only in year O. Calculate the total NPV of the project(s)...
-
Let x = (x1. . . .xn)T be an eigenvector of A belonging to λ. If |xi| = ||x||, show that (a) (b) _ ail laul (Gerschgorin's theorem) fil ti
-
1) Predict the organicproduct formed when BzCl reacts with cyclohexanol. BzCl = benzoylchloride. 2) Provide the majororganic product of the reaction below. 3) Draw the structureof the product formed...
-
A fan delivers 640 ft 3 /min (CFM) of air. If the density of the air is 1.20 kg/m 3 , compute the mass flow rate in slugs/s and the weight flow rate in lb/h.
-
A large blower for a furnace delivers 47000 ft 3 /min (CFM) of air having a specific weight of 0.075 lb/ft 3 . Calculate the weight flow rate and mass flow rate.
-
A furnace requires 1200 lb/h of air for efficient combustion. If the air has a specific weight of 0.062 lb/ft 3 , compute the required volume flow rate.
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


