Use the electronegativity values in the chart on page 189 to calculate EN and predict whether the
Question:
Use the electronegativity values in the chart on page 189 to calculate ΔEN and predict whether the bonds (Ba—Cl, O—O, and Si—O) are covalent, polar covalent, or ionic.
SiO2
Data from Page 189
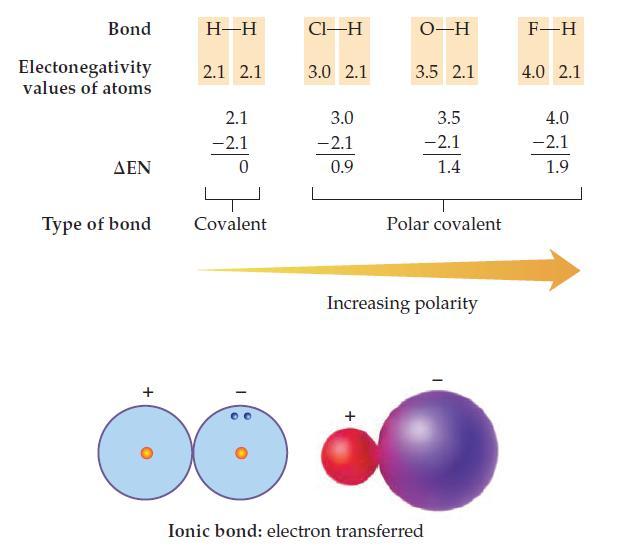
Transcribed Image Text:
Bond Electonegativity values of atoms ΔΕΝ Type of bond + H-H 2.1 2.1 2.1 -2.1 0 Covalent CI-H 3.0 2.1 3.0 -2.1 0.9 O-H 3.5 2.1 3.5 -2.1 1.4 Polar covalent Increasing polarity Ionic bond: electron transferred F-H 4.0 2.1 4.0 -2.1 1.9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate EN and predict the type of bond in SiO2 we will use the same steps as before Calculatin...View the full answer

Answered By

Allan Simiyu
I am an adroit Writer. I am a dedicated writer having worked as a writer for 3 years now. With this, I am sure to ace in the field by helping students break down abstract concepts into simpler ideas.
5.00+
8+ Reviews
54+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Use the electronegativity values in the chart on page 189 to calculate EN and predict whether the bonds (BaCl, OO, and SiO) are covalent, polar covalent, or ionic. O 3 Data from Page 189 Bond...
-
Use the electronegativity values in the chart on page 189 to calculate EN and predict whether the bonds (BaCl, OO, and SiO) are covalent, polar covalent, or ionic. BaCl 2 Data from Page 189 Bond...
-
Use the electronegativity values in Figure 1.14 to predict which of the indicated bonds in each of the following sets is more polar. Tell the direction of the polarity in each. (a) ClOCH 3 or ClOCl...
-
Determine the magnitude of the projection of the moment cause by the force about the a a axis. OKAY, SO I DONT UNDERSTAND HOW TO GET "R" BECAUSE THE FORCE IS MEASURED ON AN AXIS AND NOT A POINT OR...
-
Due to an increase in recent employee layoffs because of economic conditions and the increased risk of workplace violence, as well as an increase in domestic restraining orders that several employees...
-
Suppose you've estimated that the fifth-percentile value at risk of a portfolio is -30%. Now you wish to estimate the portfolio's first-percentile VaR (the value below which lie 1% of the returns)....
-
16-15. Cul es la diferencia entre los mayoristas mercantiles y los agentes ?
-
Do some reading in periodicals and/or on the Internet to find out more about the Sarbanes-Oxley Acts provisions for companies. Select one of those provisions, and indicate why you think financial...
-
Paper 3 Retirement planning-Have you started, what have you done well in planning? What mistakes have you made? What concerns do you have? Do you want to work in retirement, etc. How are you planning?
-
Without knowing electronegativity values, a student claims BaCl 2 is more ionic than BeCl 2 . All she has access to is a periodic table. How does she know she is right?
-
Draw a dot diagram for NO + .
-
A client invests $500,000 in a bond fund projected to earn 7 percent annually. Estimate the value of her investment after 10 years?
-
Micro-Brush requires a new component for their laptop cleaning machines. The company must decide whether to make or buy them. If it decides to make them. Should it use process A or process B? Use a...
-
Moving from a fee-for-service to a managed care delivery system set up a series of expectations (page 421). How many of these expectations are realistic? How many have been realized?
-
2. A 55 kg human is shot out the end of a cannon with a speed of 18 m/s at an angle of 60. Ignore friction and solve this problem with energy conservation. As he exits the cannon, find: a. horizontal...
-
Theoretical Background: Information Assurance (IA) architecture also known as security architecture is about planning, integrating and continually monitoring the resources of an organization so they...
-
AZCN recommends Microsoft Lens or Adobe Scan; download one of these to yo phone via your phone's app store 2. Place the document you want to scan on a flat, well-lit surface. Make sure the document...
-
The following polar equations are represented by six graphs in Figure 12. Match each graph with its equation. a. r = sin 3θ + sine2 2θ b. r = cos 2θ + cos2...
-
A consumer magazine is evaluating five brands of trash compactors for their effectiveness in reducing the volume of typical household products that are discarded. In the experiment, each block...
-
When 4 He is cooled below 2.17 K it becomes a superfluid with unique properties such as a viscosity approaching zero. One way to learn about the superfluid environment is to measure the...
-
Calculate the vibrational partition function for H 35 Cl ( = 2990 cm 1 ) at 300 and 3000. K. What fraction of molecules will be in the ground vibrational state at these temperatures?
-
Determine the rotational partition function for I 35 Cl (B = 0.114 cm 1 ) at 298 K.
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


