Using dots to represent potassiums 19 electrons, fill the subshells in the diagram below to arrive at
Question:
Using dots to represent potassium’s 19 electrons, fill the subshells in the diagram below to arrive at the ground-state energy- level diagram for the potassium atom. Select the diagram that gives an answer consistent with the periodic table.
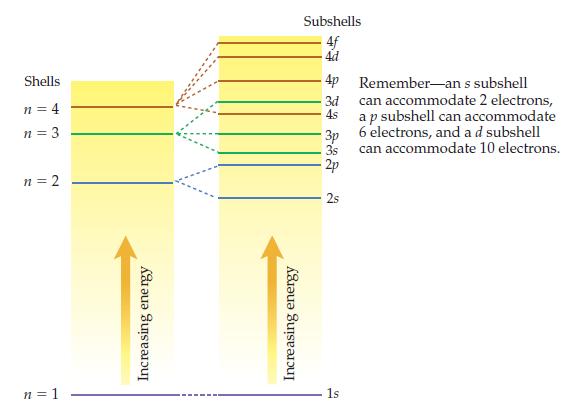
Transcribed Image Text:
Shells n = 4 n = 3 n = 2 n = 1 Increasing energy Increasing energy Subshells 4f 4d 4p 3d 4s 3p 3s -2p 2s 1s Remember-an s subshell can accommodate 2 electrons, a p subshell can accommodate 6 electrons, and a d subshell can accommodate 10 electrons.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Shells n 4 n 3 n ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
MAX DIVISOR TREE Let a tree exists with the root value N. The property of this tree is that each of its nodes branches out to the nodes with a value equal to one of its divisors (except 1 and the...
-
Why is it necessary to estimate the stage or degree of completion of work in process at the end of the accounting period under the process cost system?
-
How should Easterbrook adapt the organizational structure of McDonald's to achieve his strategic plan?
-
E10.10. Free Cash Flow for Kimberley-Clark Corporation (Medium) Below are summary numbers from reformulated balance sheets for 2007 and 2006 for Kimberly-Clark Corporation, the paper products...
-
1. Why install an ERP? 2. Why not install an ERP? 3. Do you try to cost-benefit justify such a system, and, if so, how? 4. Are there corporate culture issues involved? 5. What degree of top...
-
7. Which of the following student loans does NOT accrue interest while you are in school? a. Subsidized federal loans b. Unsubsidized federal loans c. Private loans d. Parent Plus loans
-
Why did Bohr split shells into subshells called s, p, d, and f? (a) To make chemistry complicated and harder for students to master. (b) To abide by the Uncertainty Principle. (c) To explain that...
-
The hydrogen atom has three other visible lines in its line spectrum: green, blue, and indigo/violet. Use the energy-level diagram for hydrogen and Table 4.1 to determine the electron jumps...
-
Jack Allen, Inc. is authorized to issue 14%, 10-year bonds payable. On January 1, 2025, when the market interest rate is 16%, the company issues $500,000 of the bonds. The bonds pay interest...
-
Jennifer purchased stock at $50 per share with a 75% initial margin requirement and a maintenance margin of 35%. How much equity per share must Jennifer contribute when the stock falls to $15 per...
-
Thinking about your present job and your "inventory"of leadership traits and characteristics, where are your strengths and weaknesses as a leader?Is being a leader desirable? If yes, what motivates...
-
You are facing a complex decision with several courses of possible action and probabilities associated with them. The current decision tree, based on the best possible estimates of probabilities and...
-
1. In what ways has Marriot proven an industry leader in the context of entrepreneurship in the hospitality industry. 2. What are the author's metrics of measuring entrepreneurial activity, and do...
-
Suppose you want to model the relationship between the interest rate, the economic growth rate and the inflation rate. what would be first model to fit explain.
-
Write the following equations in spherical coordinate form. a. x2 + y2 + z2 = 4 b. 2x2 + 2y2 - 2z2 = 0 c. x2 - y2 - z2 = 1 d. x2 + y2 = z
-
Discuss whether responsible human resources management should apply different standards for the home company and suppliers, for developed countries and developing countries, and for large companies...
-
Singlet and triplet carbenes exhibit different properties and show markedly different chemistry. For example, a singlet carbene will add to a cis-disubstituted alkene to produce only...
-
Electron-donating groups on benzene promote electrophilic aromatic substitution and lead preferentially to so-called ortho and para products over meta products, whereas electron-withdrawing groups...
-
Aromatic molecules such as benzene typically undergo substitution when reacted with an electrophile such as Br 2 , whereas alkenes such as cyclohexene most commonly undergo addition: What is the...
-
Ted and his partners have contracted to purchase the franchise nights worth 561 000 to open and operate a specialty pizza restaurant called Popper with a renewable agrement, the partners have agreed...
-
Your answer is partially correct. Martin Company's chief financial officer feels that it is important to have data for the entire quarter especially since their financial forecasts indicate some...
-
Kellog Corporation is considering a capital budgeting project that would have a useful life of 4 years and would love testing 5156.000 in equipment that would have zeto salvage value at the end of...

Study smarter with the SolutionInn App


