Using the EMF series on page 391, decide which of the following redox reactions is spontaneous. Explain
Question:
Using the EMF series on page 391, decide which of the following redox reactions is spontaneous. Explain your answer.
(a) 3Ag + Au3+ → Au + 3 Ag+
(b) Au + 3Ag+ → 3Ag + Au3+
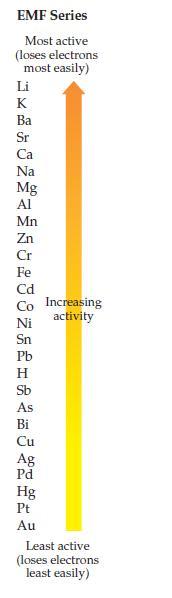
Transcribed Image Text:
EMF Series Most active (loses electrons most easily) Li HKkxQMSA MAGRGOMADHA &斑Q8HL加 Ba Sr Ca Na Mg Al Mn Zn Cr Fe Cd Co Increasing Ni Sn Pb Н Sb As Bi Cu Ag Pd Hg Pt Au activity Least active (loses electrons least easily)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 3Ag Au3 Au 3 Ag Spontaneous Explanation The EMF series shows the standard reduction potentials of ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Using the EMF series on page 391, decide which of the following redox reactions is spontaneous. Explain your answer. (a) 3K + Al 3 + Al + 3K + (b) Al + 3K + 3 K + Al 3 + EMF Series Most active...
-
Suppose gold were not available for a wedding ring, but you still wanted a ring that would last forever and not corrode. According to the EMF series on page 391, what would be a good alternative...
-
A plumber's handbook states that you should not connect a brass pipe directly to a galvanized steel pipe because electrochemical reactions between the two metals will cause corrosion. The handbook...
-
explain the term " system development" and describe the steps involved in system development
-
In the long run, the normal selling price must be set high enough to cover what factors?
-
Amanda Reiss had completed her residency in ophthalmology in Portland, Oregon, and was moving to Phoenix, Arizona, to start her practice. She began looking for office space and met with a leasing...
-
Explain the motives for FDI and collaborative ventures. LO1
-
A water pipe is connected to a double-U manometer as shown in Fig. P1116E at a location where the local atmospheric pressure is 14.2 psia, determine the absolute pressure at the center of the pipe....
-
Suppose you obtain a 25-year mortgage loan of $191,000 at an annual interest rate of 8.1%. The annual property tax bill is $967 and the annual fire insurance premium is $485. Find the total monthly...
-
You are trapped on a desert island with plenty of water (both fresh and salt), a drinking glass, some wire, a radio, and no batteries. You do have a tin cup, a tube of toothpaste containing stannous...
-
What happens when you place a less active metal in a solution of ions of a more active metal?
-
Which of the following constitutes easy money policy and is carried out directly by the Fed? a. The sale of Treasury securities b. A decrease in the federal funds rate c. A decrease in the discount...
-
Menlo Company distributes a single product. The companys sales and expenses for last month follow: Total Per Unit Sales $ 308,000 $ 20 Variable expenses 215,600 14 Contribution margin 92,400 $ 6...
-
Dr. Solo is preparing a single journal entry for December 31, 2022. The bank statement shows a balance of $10,500 on that day. Three checks were made out on that day: one for $250 for medical...
-
Predicting Gender A study addressed the issue of whether pregnant women can correctly predict the gender of their baby. Among 104 pregnant women, 57 correctly predicted the gender of their baby...
-
Chamberson Medical Center is comparing their cash flow statements for 2022 to 2021. On the following cash flow form, what would be the cash and cash equivalents for the beginning of the year for...
-
What concept is important for effective planning and can be seen in various fields, including business and politics?
-
Let A be an m n matrix with m > n. Let b Rm and suppose that N(A) = {0}. (a) What can you conclude about the column vectors of A? Are they linearly independent? Do they span Rm? Explain. (b) How...
-
The unadjusted trial balance of Secretarial Services is as follows: SECRETARIAL SERVICES Unadjusted Trial Balance as at 31 December 2017 Account Debit Credit Cash at bank Office supplies Prepaid...
-
An object is located at a distance s 0 from a spherical mirror of radius R.Show that the resulting image will be magnified by an amount R MT = 2s, + R
-
Design a little dentists mirror to be fixed at the end of a shaft for use in the mouth of some happy soul. The requirements are (1) that the image be erect as seen by the dentist and (2) that when...
-
An LED 0.60 cm tall is on the central axis 30.0 cm in front of a convex spherical mirror. If the radius of curvature of the mirror is 12.0 cm determine the location of the image, describe it, and...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


