Write the equilibrium constant expression for: (a) SiC14 (1) + 2 HO(g) (b) H(g) + CO(g)
Question:
Write the equilibrium constant expression for:
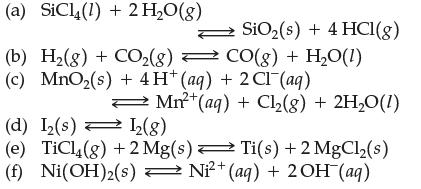
Transcribed Image Text:
(a) SiC14 (1) + 2 H₂O(g) (b) H₂(g) + CO₂(g) — CO(g) + H₂O(1) (c) MnO₂(s) + 4H* (aq) + 2 Cl(aq) SiO₂ (s) + 4 HCl(g) Mn²+ (aq) + Cl₂(g) + 2H₂O(1) (d) I₂(s) 1₂(8) (e) TiCl4(g) +2 Mg(s) Ti(s) + 2MgCl₂(s) 2+ (f) Ni(OH)₂(s) Ni²+ (aq) + 2 OH (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The equilibrium constant expressions for the reacti...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Consider the gas-state reaction (a) Write the equilibrium constant expression for the reaction. (b) Write the reaction in reverse. (c) Write the equilibrium constant expression for the reverse...
-
(a) Write the equilibrium constant expression for the reaction (b) How would the equilibrium be affected if PbI 2 (s) were added? (c) How would the equilibrium be affected if Pb(NO 3 ) 2 (s) were...
-
Compare your equilibrium constant expressions from Problem 14.48(a) and Problem 14.49. (a) Explain how the value of K eq changes for a reaction when you double the reaction (when you multiply it...
-
Mansfield Congregational Church (MCC) is a small organization in Connecticut with only seventeen employees. Though its funds are dwindling, the MCC wants to hire a full-time maintenance employee to...
-
Briefly define return on assets and what it measures.
-
What is the primary advice Mr. Buffett provides in his interview?
-
An increase in a firms growth rate _________ the PEG ratio and _________ the P/S ratio.
-
The October 31 bank statement of Wollastons Healthcare has just arrived from State Bank. To prepare the bank reconciliation, you gather the following data: a. The October 31 bank balance is $5,580....
-
Farmer Company purchased equipment on January 1, Year 1 for $81,000. The machines are estimated to have a 5-year life and a salvage value of $10,000. The company uses the straight-line method. At the...
-
For an endothermic reaction, will the equilibrium constant increase, decrease, or stay the same as the temperature of the reaction mixture increases? Explain your answer.
-
When the reaction N 2 (g) + O 2 (g) 2NO(g) is run at 2000 C, appreciable amounts of reactants and product are present at equilibrium. (a) A sealed 2.00-L container at 2000 C is filled with 1.00 mole...
-
In Chapter 4, you were asked to determine the time required for an aluminum extrusion to cool to a maximum temperature of 40C. Repeat these calculations, but determine the convection heat transfer...
-
What is Computer Programming and How to Become a Computer Programmer?
-
What Do Programmers Do All Day?
-
How Do You Become a Computer Programmer?
-
Introduction to Accounting - Meaning, Objectives Fundamentals of Accounting
-
The domain of chemical reaction engineering consists of all chemical transformations (and that includes biological) of starting materials, derived from non-renewable and renewable resources, into a...
-
The U.S. Army Materiel Command was preparing final arrangements to move its Mil3 Full-Tracked Armored Personnel Carrier from various subcontractors' manufacturing facilities to intermediate storage...
-
Solve the relation Exz:Solve therelation ne %3D
-
For the tank shown in Fig. 3.21, determine the reading of the bottom pressure gage in psig if the top of the tank is vented to the atmosphere and the depth of the oil h is 28.50 ft. Air Oil (sg =...
-
For the tank shown in Fig. 3.21, determine the reading of the bottom pressure gage in psig if the top of the tank is sealed, the top gage reads 50.0 psig, and the depth of the oil h is 28.50 ft. Air...
-
For the tank shown in Fig. 3.21, determine the reading of the bottom pressure gage in psig if the top of the tank is sealed, the top gage reads -10.8 psig, and the depth of the oil h is 6.25 ft. Air...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


