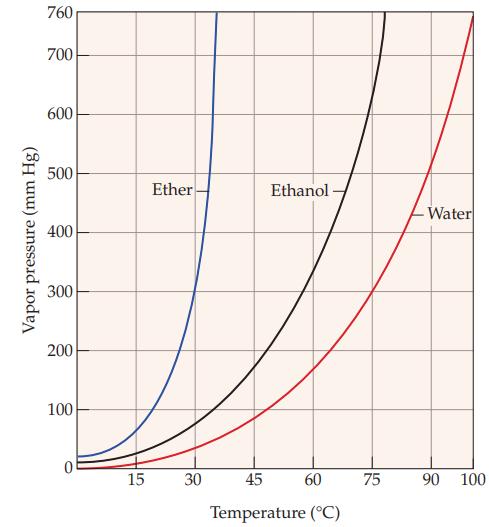
Refer to Figure 11.5 and determine the boiling point of water at an elevation where the atmospheric
Question:
Refer to Figure 11.5 and determine the boiling point of water at an elevation where the atmospheric pressure is 0.500 atm.
Figure 11.5
Transcribed Image Text:
Vapor pressure (mm Hg) 760 700 600 500 400 300 200 100 0 15 Ether 30 Ethanol- 45 60 Temperature (°C) 75 Water 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Figure 115 shows the vapor pressure of water ethanol a...View the full answer

Answered By

Munibah Munir
I've done MS specialization in finance’s have command on accounting and financial management. Forecasting and Financial Statement Analysis is basic field of my specialization. On many firms I have done real base projects in financial management field special forecasting. I have served more than 500 Clients for more than 800 business projects, and I have got a very high repute in providing highly professional and quality services.I have capability of performing extra-ordinarily well in limited time and at reasonable fee. My clients are guaranteed full satisfaction and I make things easy for them. I am capable of handling complex issues in the mentioned areas and never let my clients down.
4.60+
467+ Reviews
648+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 11.5 and determine the boiling point of ethanol at an elevation where the atmospheric pressure is 0.500 atm. Figure 11.5 Vapor pressure (mm Hg) 760 700 600 500 400 300 200 100 0 15...
-
The temperature at which water boils (the boiling point) depends on elevation: The higher the elevation, the lower the boiling point will be. At sea level, water boils at 212F; at an elevation of...
-
Use Figure 12.28 to estimate the boiling point of water at an external pressure of 200 torr. (a) 66 C (b) 84 C (c) 100 C (d) 0 C Vapor pressure (torr) 800- 760 600- 400- 200 0- 0 34.6 C Diethyl ether...
-
The statement of financial position of Kingbird Limited follows for the current year, 2020: KINGBIRD LIMITED Statement of Financial Position December 31, 2020 Current assets $135,660 Current...
-
Pop Corporation acquired a 70 percent interest in Stu Corporation on January 1, 2011, for $1,400,000, when Stu's stockholders' equity consisted of $1,000,000 capital stock and $600,000 retained...
-
Frolin Chemicals Ltd produces FDN. The standard ingredients of 1kg of FDN are: 0.65 kg of ingredient F ................................@ ?4.00 per kg0.30 kg of ingredient D...
-
From the following balances extracted from the books of Nilgiris Departmental Store as on 31.12.2001, prepare a trial balance: Gopals capital 7000 Purchases 8000 Rent paid 240 Gopals drawings 1200...
-
Suppose that Executive Aviation discovers that Air Ruidoso has sufficient assets in one of its bank accounts to pay the past-due amount. How might Executive Aviation attempt to obtain access to these...
-
In case of a not-for-profit provider, when social value is considered, the total net present value (TNPV) of a project can be expressed as the sum of its economic value (i.e., conventional NPV) and...
-
Draw the electron dot formula for H 2 S. How many nonbonding electron pairs are in a hydrogen sulfide molecule? H S
-
Calculate the percentage of water in each of the following hydrates. (a) SrCl 2 6 H 2 O (b) K 2 Cr 2 O 7 2 H 2 O (c) Co(CN) 3 3 H 2 O (d) Na 2 CrO 4 4 H 2 O.
-
Write the following method that returns the sum of all numbers in an ArrayList: public static double sum(ArrayList list) Write a test program that prompts the user to enter 5 numbers, stores them in...
-
Systems thinking is all about solving problemsin organizations, world situations, and even our personal lives. But it is not just a procedure; it is a different way of approaching problems. Our...
-
How would I display the following 3 principles in an entertaining infographic? Be very specific . Principle 1: Employee Engagement and Motivation Drawing from the Human Relations Movement theory and...
-
Shown below is a cross section of tubular member which is subjected to a torque T= 5.5 kN-m. It has a length L-3.0-m and the material shear modulus G=27 GPa. Dimensions: b=150 mm, h= 100 mm and t= 8...
-
The hip roof shown in the below Figure 2 is constructed of 2x10 rafters spaced 16 inches on center. The hip rafters are 1 -inch-wide by 12-inch-high GLBs. The roof has a slope of 4:12. Prepare a list...
-
2. Estimate the populations of Fargo, ND and Bismarck, ND in years of 2040 and 2050. Select a single value of population that you would use for design purposes in each year. You need to specify and...
-
An engineer decided to make a careful analysis of the cost of fire insurance for his $200,000 home. From a fire rating bureau he found the following risk of fire loss in any year. Outcome Probability...
-
A researcher reports a significant two-way between-subjects ANOVA, F(3, 40) = 2.96. State the decision to retain or reject the null hypothesis for this test.
-
The presidents executive jet is not fully utilized. You judge that its use by other officers would increase direct operating costs by only $20,000 a year and would save $100,000 a year in airline...
-
One measure of the effective tax rate is the difference between the IRRs of pretax and after tax cash flows, divided by the pretax IRR. Consider, for example, an investment I generating a perpetual...
-
We warned that equivalent annual costs should be calculated in real terms. We did not fully explain why. This problem will show you. Look back to the cash flows for machines A and B (in Choosing...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...
-
BE13.2 (LO 1), AP An inexperienced accountant for Silva Corporation showed the following in the income statement: net income \$337,500 and unrealized gain on availablefor-sale securities (before...

Study smarter with the SolutionInn App


