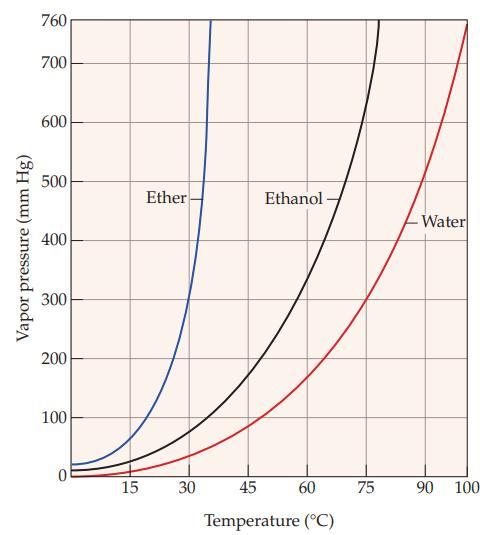
Refer to Figure 11.5 and estimate the vapor pressure of ether at each of the following temperatures:
Question:
Refer to Figure 11.5 and estimate the vapor pressure of ether at each of the following temperatures:
(a) 15 °C
(b) 30 °C
Figure 11.5
Transcribed Image Text:
Vapor pressure (mm Hg) 760 700 600 500 400 300 200 100 0 15 Ether 30 Ethanol- 45 60 Temperature (°C) 75 -Water 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The vapor pressure of ether at 15 C is approximately 300 mmHg ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 11.5 and estimate the vapor pressure of ethanol at each of the following temperatures: (a) 45 C (b) 60 C Figure 11.5 Vapor pressure (mm Hg) 760 700 600 500 400 300 200 100 0 15 Ether...
-
The vapor pressure of water at various temperatures follows: (a) Draw a scatter diagram of these data. What type of relationship seems appropriate in relating y to x? (b) Fit a simple linear...
-
The vapor pressure of 1-chlorotetradecane at several temperatures is tabulated here. (a) Use two-point linear interpolation to estimate the value of p* at T = 185 o C. (b) Write a computer subroutine...
-
Explain what you would assume the yield curve would look like during economic expansion and why.
-
Cite the conditions under which you would expect the balance of an equity investment account on a balance sheet date subsequent to acquisition to be equal to the underlying book value represented by...
-
Explain why there has been such a reduction in the number of work stoppages?
-
Unusual Values A person lives in a household with three dogs and claims that having three dogs is not unusual. Use the information in Exercise 27 to determine if this person is correct. Explain your...
-
On June 1, 2015, the City of Cape May authorized the construction of a police station at an expected cost of $250,000. Financing will be provided through transfers from a Special Revenue Fund. The...
-
1 2. 3. 4. Sandhill Company manufactures its product, Vitadrink, through two manufacturing processes: Mixing and Packagine. The following transactions were completed during October, the company's...
-
What is the general relationship between the boiling point of a liquid and its vapor pressure?
-
What is the general relationship between the vapor pressure of a liquid and its temperature?
-
Which type of radiation cannot penetrate human skin and requires heavy clothing as minimum protective shielding?
-
Low Desert Pottery works makes a variety of pottery products that it sells to retailers. The company uses a job-order costing system in which departmental predetermined overhead rates are used to...
-
ASSESSMENT CPCCBC5002A Monitor costing systems on medium rise building and construction projects Please provide answer to Part 2 - Monitor expenditure for a medium-rise project as per below...
-
Questions 6-8 refer to the same problem A sinusoidal wave with wavelength 2 m and amplitude 5 mm is traveling along the x axis. The wave is traveling in the -x direction at a speed of 2m/s At t = Os,...
-
Consider a circuit where one or more capacitors is discharged through a light bulb filament with a resistance of 3.0 0.3 . Assume that the resistance of the filament is constant (to within the stated...
-
3. For a vibrating string of length with fixed ends, each mode of vibration can be written as where wk ux(x, t) = M* sin(wxt + k) sin(x) and Mk, Ok are determined by initial conditions. For all k >...
-
For the following diagram, compute F. 30 30 30 30 30 30 25 20 15 10 0-23 4 56-891011 1-10%
-
A report from the college dean indicates that for the previous semester, the grade distribution for the Department of Psychology included 135 As, 158 Bs, 140 Cs, 94 Ds, and 53 Fs. Determine what kind...
-
Which of the following features would increase the value of a corporate bond? Which would reduce its value? a. The borrower has the option to repay the loan before maturity. b. The bond is...
-
The shareholders of the Pickwick Paper Company need to elect five directors. There are 200,000 shares outstanding. How many shares do you need to own to ensure that you can elect at least one...
-
Can you think of any new kinds of security that might appeal to investors? Why do you think they have not been issued?
-
explain the concept of Time Value of Money and provide and example. In addition to your discussion, please explain the differences between Stocks and Bonds
-
Wildhorse Inc. has just paid a dividend of $3.80. An analyst forecasts annual dividend growth of 9 percent for the next five years; then dividends will decrease by 1 percent per year in perpetuity....
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...

Study smarter with the SolutionInn App


