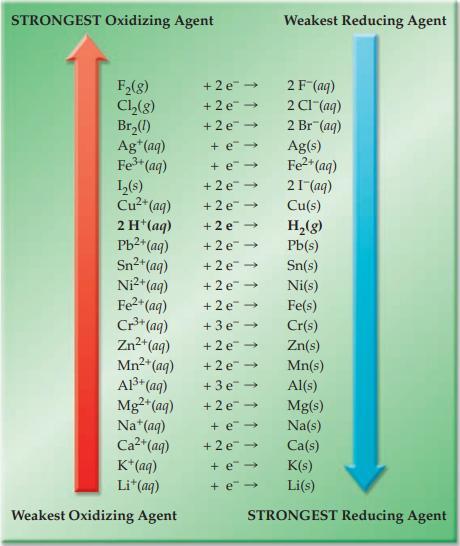
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater
Question:
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be reduced.
(a) Pb2+(aq) or Zn2+(aq)
(b) Fe3+(aq) or Al3+(aq)
(c) Ag+(aq) or I2(s)
(d) Cu2+(aq) or Br2(l)
Figure 17.4
Transcribed Image Text:
STRONGEST Oxidizing Agent F₂(8) Cl₂(g) Br₂(1) Ag+ (aq) Fe³+ (aq) L₂(s) Cu²+(aq) 2 H+ (aq) Pb²+ (aq) Sn²+ (aq) Ni²+ (aq) Fe²+ (aq) Cr³+ (aq) Zn²+(aq) Mn²+ (aq) Al³+ (aq) Mg²+ (aq) Na*(aq) Ca²+ (aq) K+ (aq) Li+ (aq) Weakest Oxidizing Agent + 2 e + 2e → +2e →>> + e → + e-> +2e → +2e →>> +2e → +2e → +2e → +2e → +2e →> +3e → +2e →> +2e → +3e → +2e → Weakest Reducing Agent + e→ +2e → + e→ + e→ 2F-(aq) 2 C1-(aq) 2 Br (aq) Ag(s) Fe²+ (aq) 21 (aq) Cu(s) H₂(g) Pb(s) Sn(s) Ni(s) Fe(s) Cr(s) Zn(s) Mn(s) Al(s) Mg(s) Na(s) Ca(s) K(s) Li(s) STRONGEST Reducing Agent
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Pb2aq or Zn2aq The standard reduction potential E for Pb2 Pb i...View the full answer

Answered By

Ajeet Singh
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students. Areas of interest: Business, accounting, Project management, sociology, technology, computers, English, linguistics, media, philosophy, political science, statistics, data science, Excel, psychology, art, history, health education, gender studies, cultural studies, ethics, religion. I am also decent with math(s) & Programming. If you have a project you think I can take on, please feel welcome to invite me, and I'm going to check it out!
5.00+
4+ Reviews
24+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be oxidized. (a) Li(s) or K(s) (b) Al(s) or Mg(s) (c) Fe 2+ (aq) or I (aq) (d) Br (aq)...
-
Which substance in each of the following pairs would you expect to have the higher boiling point? Explain why. (a) Ne or Xe, (b) CO2 or CS2, (c) CH4 or Cl2, (d) F2 or LiF, (e) NH3 or PH3
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) B or Al (b) Na or K (c) Mg or Ba (d) H or Fe. Periodic...
-
Refer to E 29 and respond to the following requirements. Data in E 2-9 Prepare the necessary adjusting entries on December 31, 2024, for the Microchip Company for each of the following situations....
-
Depreciation information for Alan Chemicals Company is given in BE9-3. Assuming the declining-balance depreciation rate is double the straight-line rate, compute annual depreciation for the first and...
-
What is the purpose of the #include directive? The basic idea of these review questions is to give you a chance to see if you have noticed and understood the key points of the chapter. You may have...
-
16. Suppose that the yield curve is given by y(t) = 0.10 0.07e 0.12t , and that the short-term interest rate process is dr(t) = ((t) 0.15r(t)) + 0.01dZ. Compute the calibrated Hull-White tree for 5...
-
Webb Corporation prepares financial statements in accordance with IFRS. Selected accounts included in the property, plant, and equipment section of the company's statement of financial position at...
-
You bought a stock one year ago for $48.97 per share and sold it today for $55.92 per share. It paid a $1.83 per share dividend today. How much of the return came from dividend yield and how much...
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs is the stronger reducing agent. (a) Cu(s) or Cr(s) (b) H 2 (g) or Cu(s) (c) Cu(s) or I (aq) (d) Cl (aq) or H 2 (g) ...
-
Chlorine can undergo a redox reaction in which it is simultaneously oxidized and reduced. Write a balanced equation for the following in a basic solution. Cl 2 (aq) ClO 2 (aq) + Cl (aq)
-
Use the eighteen rules of inference to derive the conclusions of the following symbolized arguments. 1. OD (Q.N) 2. (NVE) DS /ODS
-
a) Describe the following concepts in the context of organizational development. b) Discuss how these concepts interrelate and support each other within an organizational framework
-
Q2. a) Analyze the importance of communication in the change management process. b) Suggests strategies that a Disaster Management Organization can employ to ensure effective communication during...
-
Q3. a) Explain the following Change Management Models
-
Q3. b) Discuss how each model can be applied in real-world organizational change scenarios.
-
In this question, you will work step-by-step through an optimization problem. A craftsman wants to make a cylindrical jewelry box that has volume, V, equal to 55 cubic inches. He will make the base...
-
Refer to the graph at right. a. Write a recursive formula for the sequence. What is the common difference? What is the value of u0? b. What is the slope of the line through the points? What is the...
-
From a medical tourist perspective, compare Shouldice with the traditional hospital in terms of the key factors of competition. Using Table 15-3, why would Shouldice attract patients from outside the...
-
On February 1, Motorsports Inc. reacquired 7,500 shares of its common stock at $30 per share. On March 15, Motorsports sold 4,500 of the reacquired shares at $34 per share. On June 2, Motorsports...
-
Using the following accounts and balances, prepare the Stockholders Equity section of the balance sheet. Seventy thousand shares of common stock are authorized, and 7,500 shares have been reacquired....
-
Using the following accounts and balances, prepare the Stockholders Equity section of the balance sheet. Seventy thousand shares of common stock are authorized, and 7,500 shares have been reacquired....
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


