Refer to the electronegativity values in Figure 12.9 and predict which of the following bonds is most
Question:
Refer to the electronegativity values in Figure 12.9 and predict which of the following bonds is most polar: H—N, H—O, or H—F.
Figure 12.9
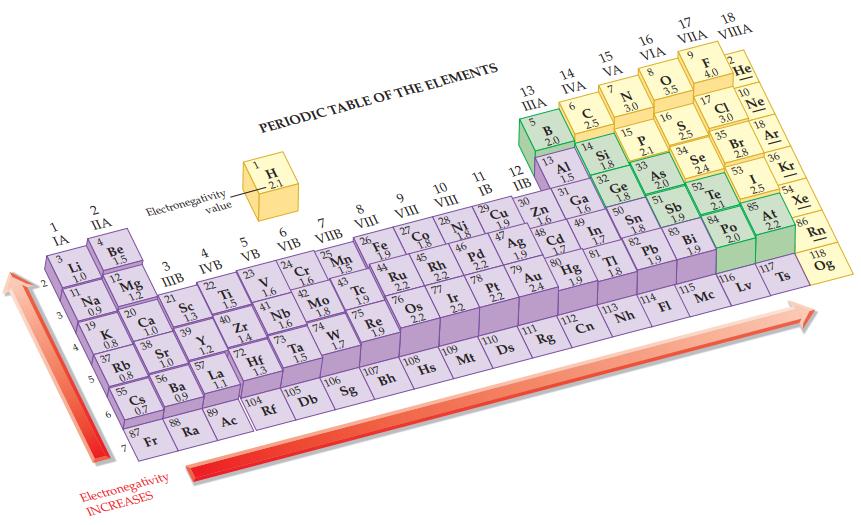
Transcribed Image Text:
1 -A 2 IA 3 3 32 283 28 Li 1.0 11 2 IIA 4 4 Na 0.9 19 5 Be 1,5 12 K 0.8 37 Mg 12 20 Rb 0.8 55 7 Ca 1.0 Electronegativity. 38 Cs 0.7 87 3 IIIB 21 Sr 1.0 56 Fr Sc 13 39 Ba 09 Electronegativity INCREASES 88 value 4 IVB 22 Y 1.2 Ra 57 Ti L 89 1.5 40 La 1.1 5 VB 23 Zr 14 72 Ac PERIODIC TABLE OF THE ELEMENTS H 2.1 V 1,6 41 Hf 13 104 6 VIB 24 Nb 1,6 73 Rf Cr 16 42 Ta 1.5 7 VIIB 25 105 Mo 1.8 74 Db 1.5 43 W 17 8 VIII 106 26 Tc 1.9 75 Sg Fe 19 44 Re 1,9 107 9 VIII 27 Ru 22 76 Bh Co 45 Os 22 108 10 VIII 28 Rh 2.2 77 Hs Ni 46 Ir 22 109 11 IB 29 Pd Mt 2.2 78 Cu 9 47 Pt 2.2 110 12 IIB 30 13 ΠΙΑ 5 79 Ds B 2.0 Zn 1.6 Ag 48 19 13 Au 111 24 14 IVA 6 AI 1.5 Cd 31 Rg _395_FS 17 180 C 2.5 14 Ga 1.6. 49 Hg 112 19 15 VA 7 Si 1.8 32 In Cn 1.7 81 N 3.0 15 Ge 1.8 TI 50 1.8 16 VIA P 2.1 113 Nh 33 Sn 1.8 82 As 16 20 51 Pb 1.9 3.5 114 17 18 VIIA VIIIA 9 S 25 FI 34 Sb 1.9 83 F 2 4.0 He Se 24 Bi 19 17 52 115 CL 3.0 35 Te 2.1 84 Mc 10 Br 2.8 53 Po 20 116 Ne 18 I 25 85 Ar Lv 36 Kr 117 54 Xe At 86 22 Rn Ts 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
HF has the greatest ...View the full answer

Answered By

Bree Normandin
Success in writing necessitates a commitment to grammatical excellence, a profound knack to pursue information, and a staunch adherence to deadlines, and the requirements of the individual publication. My background comprises writing research projects, research meta-analyses, literature reviews, white paper reports, multimedia projects, reports for peer-reviewed journals, among others. I work efficiently, with ease and deliver high-quality outputs within the stipulated deadline. I am proficient in APA, MLA, and Harvard referencing styles. I have good taste in writing and reading. I understand that this is a long standing and coupled with excellent research skills, analysis, well-articulated expressions, teamwork, availability all summed up by patience and passion. I put primacy on client satisfaction to gain loyalty, and trust for future projects. As a detail-oriented researcher with extensive experience surpassing eight years crafting high-quality custom written essays and numerous academic publications, I am confident that I could considerably exceed your expectations for the role of a freelance academic writer.
5.00+
7+ Reviews
21+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the electronegativity values in Figure 12.9 and predict which of the following bonds is least polar: CN, CO, or CI. Figure 12.9 1 IA 2 3 3 Li 1.0 11 4 2 IIA 4 Na 0.9 19 10 5 Be 1,5 12 K 0.8...
-
Use the electronegativity values in Figure 1.14 to predict which of the indicated bonds in each of the following sets is more polar. Tell the direction of the polarity in each. (a) ClOCH 3 or ClOCl...
-
R. S. Mulliken proposed that the electronegativity (EN) of an atom is given by where E i and E ea are the ionization energy and electron affinity of the atom, respectively. Using the electron...
-
Chemistry A one-electron atom is an atom with Z protons in the nucleus and one electron. For example, Z = 2 for helium and Z = 3 for lithium. Use our class discussion of the allowed radii and...
-
Eli, Joe, and Ned agree to liquidate their consulting practice as soon as possible after the close of business on July 31, 2011. The trial balance on that date shows the following account balances:...
-
APICS is a professional organization for operations and supply chain management. Find out if there is a local APICS chapter in your area. Attend a meeting and write a summary of the speakers...
-
What is a standard? What role do standards play in project quality management? AppendixLO1
-
Emerson St. Paul Book Shops accounts at June 30, 2015, included the following unadjusted balances: Merchandise Inventory .........$ 5,400 Cost of Goods Sold ......... 40,300 Sales Revenue ..............
-
Discrete Math- Design Hi could you please explain to me all the steps generously with a HANDWRITTEN NOTES. Thanks that is all i got, if you have a difficulty it is oki, just close it, so someone with...
-
What is the molecular shape for a molecule of H 2 S? (a) Bent (b) Linear (c) Tetrahedral (d) Trigonal pyramidal (e) None of the above.
-
Calculate the electronegativity difference and apply delta notation to the bond between carbon and oxygen, CO.
-
Identify ways in which activity-based management can achieve improvements in an organization. LO9
-
If you are a Super Coffee company and want to partner with Influencers on Instagram. How to find them? Influencer suggestions? How much to pay them? Who are they? Your budget is $250,000 So...
-
As the Customer Support Manager in the Fast-Moving Consumer Good (FMCG) sector with the ABC Corporation you are expected to address major grievances of customers from our product line and special...
-
An agent for positive change can be defined as someone who has the capability to influence and motivate those around them to accomplish whatever shared task needed to achieve a common goal, these...
-
Identify stress-reduction techniques used in organizations (e.g., wellness programs) Evaluate the impact of personal and work-related stress on performance Explain the relationship between...
-
Company: Viventium Explain which metrics you recommend tracking and why. For example, "these are the 3 to 5 metrics I've chosen; I'm tracking this because it does X, thereby bringing me closer to...
-
A corporation with a 34% income tax rate is considering the following investment in research equipment and has projected the benefits as follows: Year Before-Tax Cash Flow...
-
One hundred pounds of water at atmospheric pressure are heated from 60F to 200F. What is the enthalpy change? The internal energy change? Why is the difference between the internal energy change and...
-
Job order costing: actual, normal, and variation from normal costing. Thanatos & Hades (T&H) is law firm that specializes in writing wills. Its job-costing system has one direct cost pool,...
-
Proration of overhead the Ride-On-Water Company (ROW) produces a line of non-motorize boats. ROW uses a normal costing system and allocates manufacturing overhead using direct manufacturing labor...
-
Job costing, accounting for manufacturing overhead, budgeted rates. The Solomon Company uses a job-costing system at its Dover, Delaware, plant. The plant has a Machining Department and a Finishing...
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...

Study smarter with the SolutionInn App


