Refer to the periodic table and state the molar mass for each of the following. (a) Aluminum,
Question:
Refer to the periodic table and state the molar mass for each of the following.
(a) Aluminum, Al
(b) Silicon, Si
(c) Arsenic, As
(d) Sulfur, S.
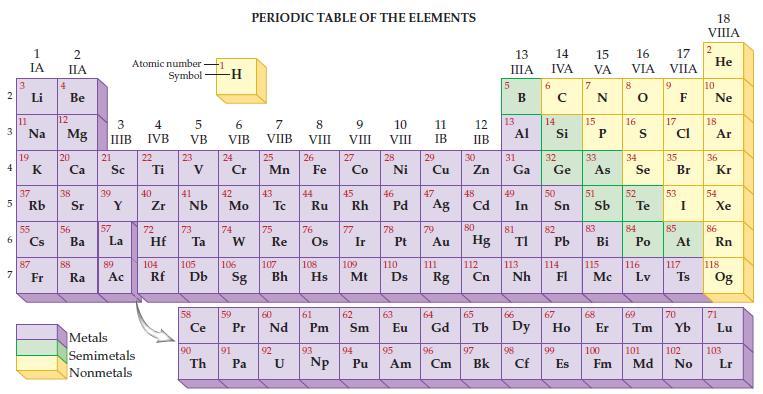
Transcribed Image Text:
2 3 4 av 6 3 7 11 1 IA 37 5 Rb Li Na 19 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Ac Atomic number Symbol Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta Ce 90 -H Th 6 VIB 24 Cr 42 Mo 74 W 59 91 PERIODIC TABLE OF THE ELEMENTS 7 VIIB Pa 25 Mn 43 75 60 Pr Nd Re 92 8 VIII U 26 44 Fe Ru 76 61 104 105 106 107 108 109 110 Rf Db Sg Bh Hs Mt D Pm 93 9 VIII Np 27 Co 77 Ir 62 Sm 10 VIII 45 46 47 Rh Pd Ag 94 28 Pu Ni 78 63 11 IB 29 95 Cu 79 Au 111 64 Eu Gd Rg 96 Am Cm 12 13 IIB 30 Zn 48 Cd 80 Hg 112 Cn 65 97 13 IIIA Bk 31 Al Ga 49 In 81 TI 113 Nh 66 98 Cf 14 15 16 IVA VA VIA 6 14 50 82 Pb 32 33 34 Ge As Se 114 67 99 E 15 Es 51 83 Bi 115 Mc 68 8 16 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 cl 35 Br 53 85 At 117 Ts 70 100 101 102 Fm Md No 18 VIIIA 2 He 10 Ne 18 Ar 36 Kr 54 Xe 86 Rn 118 Og 71 Lu 103 Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
The molar mass of an element is the mass of one mole of atom...View the full answer

Answered By

PU Student
cost accounting
financial accounting
auditing
internal control
business analyst
tax
i have 3 years experience in field of management & auditing in different multinational firms. i also have 16 months experience as an accountant in different international firms. secondary school certification.
higher secondary school certification.
bachelors in mathematics.
cost & management accountant
4.80+
4+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and state the highest energy sublevel for each of the following elements. (a) He (b) K (c) U (d) Pd (e) Be (f) Co (g) Si (h) Pt. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94...
-
Refer to the periodic table and state the highest energy sublevel for each of the following elements. (a) H (b) Na (c) Sm (d) Br (e) Sr (f) C (g) Sn (h) Cs. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94...
-
Refer to the periodic table and state the molar mass for each of the following. (a) Gallium, Ga (b) Germanium, Ge (c) Antimony, Sb (d) Selenium, P. 2 3 4 AD 6 3 7 11 1 IA 37 5 Rb Li Na 19 55 87 Fr 4...
-
Suppose the following model describes the relationship between annual salary (salary) and the number of previous years of labor market experience (exper): log(salary) = 10.6 + .027 exper. (i) What is...
-
Presented below are three different lease transactions that occurred for Manitoba Inc. in 2012. Assume that all lease contracts start on January 1, 2012. In no case does Manitoba receive title to the...
-
In a survey of 1035 U.S. adults, 745 say they want the U.S. to play a leading or major role in global affairs. Let p be the population proportion for the situation. (a) Find point estimates of p and...
-
Comparing Renting and Buying. Based on the following data, would you recommend buying or renting? Rental Costs Buying Costs Annual rent, $7,380 Insurance, $145 Security deposit, $650 Annual mortgage...
-
Information for three costs incurred at Boole Manufacturing in the first quarter follows: Required Plot each cost, making the vertical axis cost and the horizontal axis units produced. Classify each...
-
The following information is available for Perry, Inc. At the end of the year, the market price of its common stock is $550 per share. Earnings per share totaled $100 and dividends per share in the...
-
TNT (trinitrotoluene) is a white crystalline substance that explodes at 240 C. Calculate the percent composition of TNT, C 7 H 5 (NO 2 ) 3 .
-
If an analysis of sugar, C x H y O z , gave 40.0% C and 6.7% H, what is the percent oxygen?
-
What constitutes overheads? LO-3
-
What are the major immediate concerns for the HR manager in Austral Group SAA when merging two different organizational cultures - in this case, Peruvian and Norwegian cultures?
-
Explain the relation between the corporate, business and functional strategies. Please produce an in-depth explanation.
-
Consider the problem of terrorism during Radical Reconstruction. If you had been an adviser to the President, how would you propose to deal with the problem? Give a minimum of TWO examples and fully...
-
describe at least one element of an Airport Master Plan. Discuss the importance of this element and how it fits into the overall Airport Master Plan document to include its processes and objectives.
-
It is suggested that Wikipedia has replaced the hardback encyclopedia books, such Encyclopedia Brittanica. What other ways do you foresee technology changing businesses that have been around for...
-
The J = 2 to 3 rotational transition in a certain diatomic molecule occurs at 126.4 GHz, where 1 GHz 109 Hz. Find the frequency of the J = 5 to 6 absorption in this molecule.
-
From a medical tourist perspective, compare Shouldice with the traditional hospital in terms of the key factors of competition. Using Table 15-3, why would Shouldice attract patients from outside the...
-
LaTour Inc. is based in France and prepares its financial statements in accordance with iGAAP. In 2010, it reported cost of goods sold of 578 million and average inventory of 154 million. Briefly...
-
Reed Pentak, finance major, has been following globalization and made the following observation concerning accounting convergence: I do not see many obstacles concerning development of a single...
-
What modifications to the conventional retail method are necessary to approximate a LIFO retail flow?
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


