Refer to the periodic table and state the molar mass for each of the following. (a) Gallium,
Question:
Refer to the periodic table and state the molar mass for each of the following.
(a) Gallium, Ga
(b) Germanium, Ge
(c) Antimony, Sb
(d) Selenium, P.
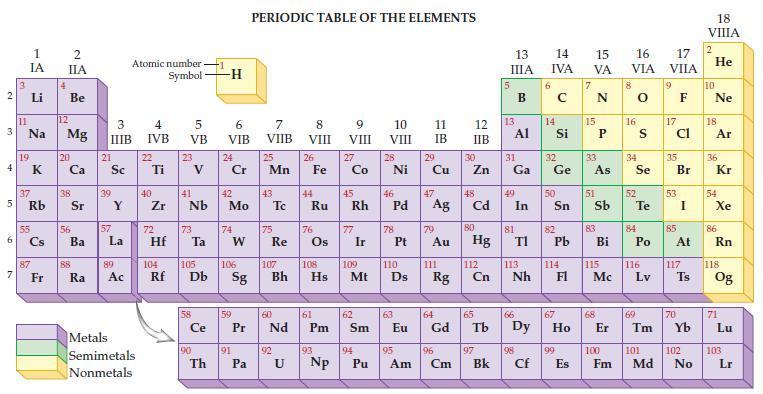
Transcribed Image Text:
2 3 4 AD 6 3 7 11 1 IA 37 5 Rb Li Na 19 55 87 Fr 4 2 IIA Be 12 Mg 20 38 56 Ba 88 Ra 3 IIIB 21 Sc 39 57 Y La 89 Atomic number Symbol Ac Metals Semimetals Nonmetals 4 IVB 22 Ti 40 Zr 5 VB 72 23 41 Nb 73 Hf Ta 104 105 Rf Db Ce 90 Th -H 6 VIB 24 Cr 42 Mo 74 W 106 Sg 59 91 PERIODIC TABLE OF THE ELEMENTS Pa 7 VIIB 25 Mn 43 75 Re 60 Pr Nd 107 Bh 92 U 8 VIII 26 44 Fe Ru 76 108 Hs 61 Pm 93 Np 9 VIII 27 Co 77 Ir 109 62 45 46 47 Rh Pd Ag Sm 10 VIII 110 Mt Ds 94 28 Pu Ni 78 63 11 IB 29 95 Cu 79 Au 111 64 Eu Gd Rg 96 Am Cm 12 13 IIB 30 Zn 48 Cd 80 Hg 112 Cn 65 97 13 IIIA Bk 31 Al Ga 49 In 81 TI 113 Nh 66 98 Cf 14 15 16 IVA VA VIA 6 14 32 Ge 50 Sn 82 Pb 114 67 99 E Es 33 51 34 As Se 83 Bi 115 Mc 68 8 16 52 S Te 84 Po 116 Lv 69 17 VIIA 9 17 cl 35 Br 53 85 At 117 Ts 70 100 101 102 Fm Md No 18 VIIIA 2 He 10 Ne 18 Ar 36 54 Kr Xe 86 Rn 118 Og 71 Lu 103 Lr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Gallium Ga Molar mass 6972...View the full answer

Answered By

Amit Choudhary
I'm new in this profession regarding online teaching but previously i used to teach students near my college. I am teaching on online platform since last year and got good support from the students. I'm teaching on platforms like chegg and vedantu and also at my home in free time.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The chemical formulas for the oxides of potassium, calcium, gallium, and germanium are, respectively, K 2 O, CaO, Ga 2 O 3 , and GeO 2 . Refer to the periodic table and predict the chemical formula...
-
Refer to the periodic table and state the molar mass for each of the following. (a) Aluminum, Al (b) Silicon, Si (c) Arsenic, As (d) Sulfur, S. 2 3 4 av 6 3 7 11 1 IA 37 5 Rb Li Na 19 55 87 Fr 4 2...
-
Refer to the periodic table and state the highest energy sublevel for each of the following elements. (a) He (b) K (c) U (d) Pd (e) Be (f) Co (g) Si (h) Pt. Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94...
-
In Exercises find the given higher-order derivative. 2 "(x) = 2 / f(x) X
-
On January 1, 2012, Zakiuddin Company purchased the following two machines for use in its production process. Machine A: The cash price of this machine was $55,000. Related expenditures included:...
-
1. Why is ethics important to doing business? 2. If all companies established a corporate ethics code, would that eliminate company financial scandals? Why or why not? For nearly 40 years, ExxonMobil...
-
E8.8. Reformulation of an Equity Statement and Accounting for the Exercise of Stock Options: Starbucks Corporation (Hard) The statement of shareholders' equity below for Starbucks Corporation, the...
-
The assembly department has the following production and cost data for the current month. Materials are entered at the beginning of the process. The ending work in process units are 70% complete as...
-
Bama Tide Inc. typically uses debtas their main source of funding and typically only finances with 30% equity. The firm's before-tax cost of debt has been estimated to be 3% while their before-tax...
-
Iron can react with chlorine gas to give two different compounds, FeCl 2 and FeCl 3 . If 0.558 g of metallic iron reacts with chlorine gas to yield 1.621 g of iron chloride, which iron compound is...
-
Calculate the number of molecules in 0.0763 mol of chlorine gas, Cl 2 .
-
Considering the expense associated with having more managers, what are some reasons why you think Starbucks decided to decrease the number of stores each district manager was responsible for, thus...
-
1. What does the phrase "cost of quality" mean? How might using this statement assist a company in addressing its quality issues? 2. What key distinctions exist between total quality human resource...
-
Does productivity in terms of output per labor our insure a company will be profitable? Why or why not? What questions should be asked to test whether productivity has increased? How do these answers...
-
How do the four Ps of marketing (product, price, promotion, place) differ in international markets?
-
Do you agree with the societal or political forces? Why or why not? Support your assertions with credible sources
-
How do the global transformational leadership models comprise a work environment that sees the need for change and embraces the new changes?Explain
-
(a) Find the cube roots of 1. (b) Explain why the n nth roots of 1 when plotted in the complex plane lie on a circle of radius 1 and are separated by an angle 2/n from one another
-
Write a declaration for each of the following: a. A line that extends from point (60, 100) to point (30, 90) b. A rectangle that is 20 pixels wide, 100 pixels high, and has its upper-left corner at...
-
Which is likely to be a more serious problem, perceptions of being under rewarded or perceptions of being over rewarded?
-
What are some managerial implications of equity theory beyond those discussed in the chapter?
-
Do you think expectancy theory is too complex for direct use in organizational settings? Why or why not?
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


