The chemical formulas for the oxides of potassium, calcium, gallium, and germanium are, respectively, K 2 O,
Question:
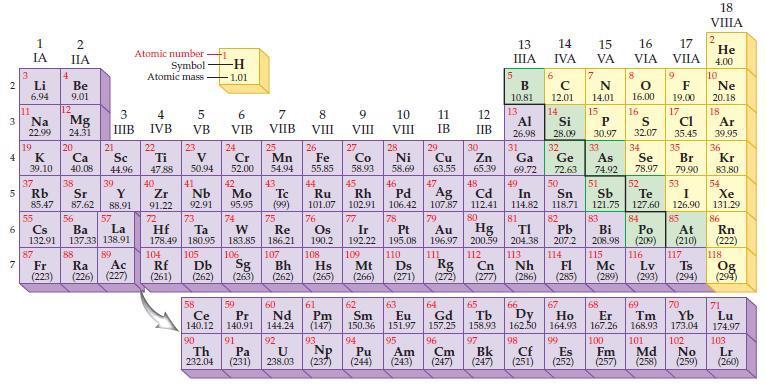
The chemical formulas for the oxides of potassium, calcium, gallium, and germanium are, respectively, K2O, CaO, Ga2O3, and GeO2. Refer to the periodic table and predict the chemical formula for each of the following compounds:
(a) Rubidium oxide
(b) Strontium oxide
(c) Indium oxide
(d) Lead oxide.
Periodic Table:
Transcribed Image Text:
2 3 4 15 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 R Rb 4 87 2 IIA Be 9.01 12 Fr (223) K Ca Sc 39.10 40.08 44.96 Mg 24.31 20 38 21 Sr Y 85.47 87.62 88.91 3 IIIB 88 39 55 56 La Cs Ba 132.91 137.33 138.91 57 89 Ra Ac (226) (227) Atomic number Symbol Atomic mass 4 IVB 22 Ti 47.88 40 Zr 2 91.22 72 5 VB 104 23 V 50.94 41 73 Hf Ta 178.49 180.95 105 Rf Db (261) (262) -H -1.01 6 VIB Nb Mo 92.91 95.95 90 24 Cr 52.00 42 74 106 Sg (263) 58 Pr Ce 140.12 140.91 91 59 7 VIIB Th Pa 232.04 (231) 25 W Re 183.85 186.21 Mn 54.94 43. Tc (99) 75 107 Bh (262) 60 Nd 144.24 92 U 238.03 8 VIII 26 Fe 55.85 76 Os 190.2 61 Pm (147) 93 Np 9 VIII (237) 10 VIII 11 IB 12 IIB 27 28 29 Zn Co Ni Cu 58.93 58.69 63.55 65.39 44 47 49 45 46 48 Ru Rh Pd Ag Cd In 101.07 102.91 106.42 107.87 112.41 114.82 77 78 80 79 Au Hg 196.97 200.59 111 112 Ir Pt 192.22 195.08 109 110 Hs Mt Ds Rg Cn (265) (266) (271) (272) (277) 108 30 5 13 ΠΙΑ B 10.81 13 6 14 IVA с 12.01 14 15 16 17 VA VIA VILA 7 N 14.01 15 66 67 62 63 64 65 Sm Eu Gd Tb Dy Ho 150.36 151.97 157.25 158.93 162.50 164.93 99 94 95 96 97 Pu Am Cm Bk (244) (243) (247) (247) 8 P 30.97 O 16.00 Al Si 31 34 53 26.98 28.09 32 33 Ga Ge As Se 69.72 72.63 74.92 78.97 50 51 52 Sn Sb Te I 118.71 121.75 127.60 126.90 82 83 84 85 TI Pb Bi Po At 204.38 207.2 208.98 (209) (210) 113 114 115 116 117 Nh Fl Mc Lv Ts (286) (285) (289) (293) (294) 81 16 9 F 19.00 17 Cl 35.45 S 32.07 35 Br 79.90 68 69 70 Er Tm Yb 167.26 168.93 173.04 100 101 102 Cf Es Fm Md No (251) (252) (257) 98 (258) (259) 18 VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39,95 36 Kr 83,80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 Lr (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a Rb 2 ...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
140+ Reviews
437+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
The formulas for the chlorides of potassium, calcium, boron, and germanium are, respectively, KCl, CaCl 2 , BCl 3 , and GeCl 4 . Using the periodic table, predict the chemical formulas for each of...
-
The formulas for the oxides of sodium, magnesium, aluminum, and silicon are, respectively, Na 2 O, MgO, Al 2 O 3 , and SiO 2 . Using the periodic table, predict the chemical formulas for each of the...
-
Arthur Korrey is developing a project to start a new professional aquatic baseball league, with teams comprised of humans, dolphins and penguins. He forecasts that net annual cash flows will be zero...
-
Solve the inequalities in Problems 4150. 7-5A < 2A + 7
-
Comparison of Public and Private Universities. Following are the operating statements for a public and private university. The operating statements have been adapted from the annual reports of a...
-
A falling liquid film within a gasliquid contactor of 1.50 m length is in contact with 100% carbon dioxide gas at 1.0 atm and 25C. The wetted surface area is 0.50 m 2 , and the liquid film thickness...
-
8-1. Market segmentation involves aggregating prospective buyers into groups that have two key characteristics. What are they?
-
Service department cost allocation is the first stage in a two-stage system. Suppose a company has a purchasing department that is responsible for buying all materials, including miscellaneous...
-
A. On 2 January 2016, Wendy Ltd purchased a machine for $55 000 plus GST with a useful life of 5 years and a residual value of $8 000. In order to keep the machine running properly, the company has...
-
Metallic sodium reacts with chlorine gas to give sodium chloride, NaCl. Predict the products formed when (a) Lithium (b) Potassium react with chlorine gas.
-
Predict the missing value (?) for each physical property listed below. The (a) Atomic radius, (b) Density, (c) Melting point are given for two of the metals in Group VIII/10. Element Ni Pd Pt Atomic...
-
Using the periodic table, predict the charges of the ions of the following elements: (a) Ga, (b) Sr, (c) As, (d) Br, (e) Se.
-
TST102 Fundamentals of Test Evaluation Lesson 17 - Assignment Assignment 1: Developmental Test Planning You are designing a developmental test to verify that the SRAW safe-arm device (SAD) arms the...
-
The Mariana snailfish (see the photo) holds the record for the world's deepest living fish. The snailfish has been found in the Mariana Trench at a depth of 26 500 feet below the water's surface. (a)...
-
A soil sample was taken from a proposed cut area in a highway construction project and sent to a soils laboratory for a compaction test, using the Standard Proctor compaction procedure. Results of...
-
Sarah agrees to sing at Joe and Sandra's June wedding and signs a contract. In April, Sarah is invited to be the top billed singer and entertainer for Carnival Cruises. It is a 2 year contract that...
-
P = 7 kN, w = 12 KN/m and angle is 32 deg Equilibrium and support reactions for beams and frames Question 2a [25 marks] Show that the following beam is statically determinate. Determine the external...
-
Ethical dilemmas often face university and college administrators as they attempt to provide students with services and products to make college life easier. For example, the University of Minnesota...
-
Suppose that fraction used = / 1.0 + 0.1Mt. for some parameter 1. Write the discrete-time dynamical system and solve for the equilibrium. Sketch a graph of the equilibrium as a function of ....
-
Andrews Inc., a greeting card company, had the following statements prepared as of December 31, 2010. Additional information: 1. Dividends in the amount of $6,000 were declared and paid during 2010....
-
Data for Andrews Inc. are presented in E23-13. Prepare a statement of cash flows using the indirect method.
-
Presented below are data taken from the records of Morgenstern Company. Additional information: 1. Held-to-maturity securities carried at a cost of $43,000 on December 31, 2009, were sold in 2010 for...
-
You are the digital marketing director for High West fashions, a regional clothing company that specializes in custom t-shirts. Your company has decided to launch an online advertising campaign that...
-
In-the-money put options will automatically get exercised at the expiration. True OR False
-
Which of the following examples of business-use property is NOT eligible for Section 1231 treatment when sold at a gain? * Sale of land held for three years. Net gain from a casualty gain on a dump...

Study smarter with the SolutionInn App


