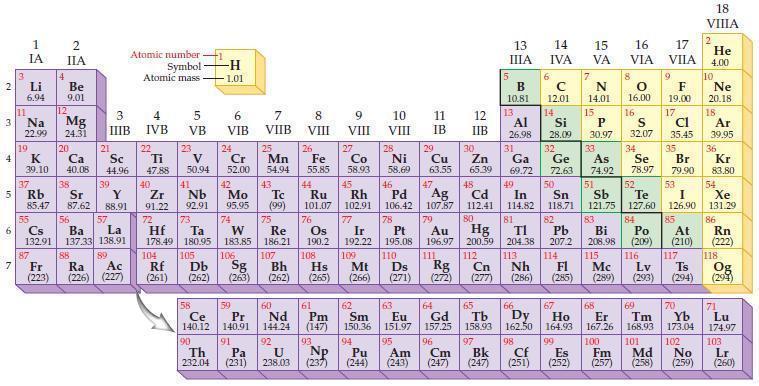
Refer to the periodic table and state the noble gas with an electron configuration identical to each
Question:
Refer to the periodic table and state the noble gas with an electron configuration identical to each of the following ions?
(a) Se2–
(b) Br–
(c) Rb+
(d) Sr2+.
Periodic Table
Transcribed Image Text:
2 3 4 5₁ 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55 4 87 2 IIA Fr (223) Be 9.01 12 K Ca Sc 39.10 40.08 44.96 Mg 24.31 38 Rb Sr Y 85.47 87.62 88.91 56 57 Cs Ba La 132.91 137.33 138.91 20 21 88 3 IIIB 39 89 Atomic number Symbol - Ac Ra (226) (227) Atomic mass 4 IVB 22 Ti 47.88 40 5 VB 23 V 50.94 41 Zr Nb 91.22 92.91 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 108 9 VIII 61 Pm (147) 27 Bh Hs Mt (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB Pt 195.08 110 29 Cu 63.55 13 IIIA 12 IIB 5 B 10.81 13 17 16 VA VIA VIIA 8 Al 26.98 6 C 12.01 14 Si 28.09 32 30 31 33 As Se Br Zn Ga Ge 65.39 69.72 72.63 74.92 78.97 79.90 14 15 IVA 7 N 14.01 15 P 30.97 16.00 83 Bi 208.98 115 16 S 32.07 34 84 Po (209) 47 48 49 50 51 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 79 Au Hg 196.97 200.59 111 112 Rg Cn (271) (272) (277) (286) (285) (289) (293) (294) 81 82 TI Pb 204.38 207.2 113 114 Nh Fl Ds Mc Lv Ts 9 116 F 19.00 Md (258) 17 Cl 35.45 35 53 I 126.90 85 At (210) 66 67 69 70 63 64 65 68 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 98 99 100 Cf Es Fm (251) (252) (257) 117 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Noble Gas for Each Ion a Se2 Krypton Kr Selenium has 34 electronsand gaining ...View the full answer

Answered By

Akash Goel
I am in the teaching field since 2008 when i was enrolled myself in chartered accountants course
Since then i have an experience of teaching of class XI, XII, BCOM, MCOM, MBA, CA CPT.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to the periodic table and state the noble gas with an electron configuration identical to each of the following ions. (a) S 2 (b) Cl (c) K + (d) Ca 2+ . Periodic Table 2 3 4 5 6 7 3 11 Li...
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) Br (b) Te 2 (c) As 3 (d) O 2 . Periodic Table: 2 3 4 10...
-
Refer to the periodic table and write the predicted electron configuration for each of the following negative ions using core notation. (a) F (b) S 2 (c) N 3 (d) I . Periodic Table: 2 3 4 10 6 3...
-
In figure, one end of a uniform beam weighing 20 x V3N is attached to a wall with a hinge. The other end is supported by a wire connected to the wall as shown. If the tension in the wire is a x 10N,...
-
1. Bankruptcy Insolvency means: a. Book value of assets is greater than liabilities b. Fair value of assets is less than liabilities c. Inability to meet financial obligations as they come due d....
-
Short-term financing is structured in many different ways. The following site from British Columbia describes different types of short-term credit. www.smallbusinessbc.ca What type of loan will suit...
-
5. What are some of the common features of the 2008 stock market crash and previous market crashesfor example, Japans in the 1990s or the Internet bubble around the turn of the millennium?
-
Children's Hospital of the King's Daughter in Norfolk, Virginia, introduced a new budgeting method that allowed the hospital's annual plan to be updated for changes in operating plans. For example,...
-
Sunland Company, a dealer in machinery and equipment, leased equipment to Sarasota, Inc., on July 1 , 2 0 2 5 . The lease is appropriately accounted for as a sales - type lease by Sunland and as a...
-
Using VSEPR theory, contrast the molecular shape of an ammonia molecule, NH3 , with that of an ammonium ion, NH4+.
-
Write out the electron configuration for each of the following nonmetal ions. (a) F (b) O 2 (c) N 3 (d) C 4 .
-
Reconsider Prob. 496. Using EES (or other) software, plot the soil temperature as a function of the distance from the earths surface as the distance varies from 0 m to 1m, and discuss the results....
-
how is lateral force(fy) determined from this data Tyre Responses 1 1 1 1 1 1.3 1.3 1.3 1.3 1.3 1.6 1.55 1.45 1.27 1.1 Fz (N) 0 400 800 1200 1500 Slip Angle (deg) Fy1 (N) Fy2 (N) Fy3 (N) 0.0 0 0 0.5...
-
(13%) Problem 8: A wire is oscillated to create a wave of the form y(x,t) = Asin(x - 30t) == The wave is reflected from a fixed end producing a reflection of the form y2(x,t) = A sin(x + 30t) The two...
-
Using the definitions of even integer and odd integer, give a proof by contraposition that this statement is true for all integers n: If 5n+3 is even, then n is odd.
-
7. Design the formwork for a wall 8-ft (2.44-m) high to be poured at the rate of 5 ft/h (1.53 m/h) at a temperature of 77F (25C). The concrete mixture will use Type I cement without retarders and is...
-
tempt in Progress The City of Minden entered into the following transactions during the year 2026. 1. A bond issue was authorized by vote to provide funds for the construction of a new municipal...
-
Mary O'Leary's company ships fine wool garments from County Cork, Ireland. Five years ago she purchased some new automated packing equipment having a first cost of $125,000 and a MACRS class life of...
-
Find a least expensive route, in monthly lease charges, between the pairs of computer centers in Exercise 11 using the lease charges given in Figure 2. a) Boston and Los Angeles b) New York and San...
-
Computing and interpreting manufacturing unit costs. Minnesota Office Products (MOP) produces three different paper products at the plant. It currently uses the following three-part classification...
-
Direct indirect fixed and variable casts, Ceramica Company manufactures three kinds of hand- painted ceramic figurines in a two-step process. The first step is automated; in the Baking Department a...
-
Classification of casts, service sector Consumer Focus is a marketing research firm that organizes focus groups for consumer-product companies. Each focus group has eight individuals who are paid $50...
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


